| |
| Names | |
|---|---|
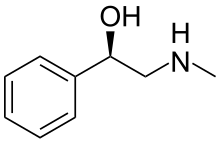
| IUPAC name
2-(Methylamino)-1-phenylethanol
| |
| Other names
N-Methylphenylethanolamine; 1-Hydroxy-1-phenyl-2-methylaminoethane; α-(Methylaminomethyl)benzyl alcohol; 2-Methylamino-1-phenylethanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H13NO | |
| Molar mass | 151.209 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 43 to 45 °C (109 to 113 °F; 316 to 318 K) (R- or S- enantiomer); 75–76 °C (racemate) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H332 | |
| P261, P264, P270, P271, P301+P312, P304+P312, P304+P340, P312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Halostachine (also known as N-methylphenylethanolamine) is a natural product, an alkaloid first isolated from the Asian shrub Halostachys caspica (synonym Halostachys belangeriana), and structurally a β-hydroxy-phenethylamine (a phenylethanolamine) related to its better-known "parent" biogenic amine, phenylethanolamine, to the adrenergic drug synephrine, and to the alkaloid ephedrine. The pharmacological properties of halostachine have some similarity to those of these structurally-related compounds, and Halostachys caspica extracts have been included as a constituent of certain OTC dietary supplements,[1] but halostachine has never been developed as a prescription drug. Although it is found in nature as a single stereoisomer, halostachine is more commonly available as a synthetic product in the form of its racemate (see below). In appearance it is a colorless solid.
- ^ "Dietary Supplements Labels Database". dietarysupplements.nlm.nih.gov. Archived from the original on 17 February 2013. Retrieved 2 February 2022.