| |

| |
| Names | |
|---|---|
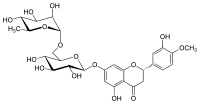
| IUPAC name
(2S)-3′,5-Dihydroxy-4′-methoxy-7-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavan-4-one
| |
| Systematic IUPAC name
(22S,42S,43R,44S,45S,46R,72R,73R,74R,75R,76S)-13,25,43,44,45,73,74,75-Octahydroxy-14-methoxy-76-methyl-22,23-dihydro-24H-3,6-dioxa-2(2,7)-[1]benzopyrana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphan-24-one | |
| Other names
Hesperetin, 7-rutinoside,[1] Cirantin, hesperidoside|heperetin, 7-rhamnoglucoside, hesperitin, 7-O-rutinoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.536 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H34O15 | |
| Molar mass | 610.565 g·mol−1 |
| Density | 1.65 ± 0.1g/mL (predicted) |
| Melting point | 262 °C |
| Boiling point | 930.1 ± 65 °C (predicted) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone is hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
Hesperidin was first isolated in 1828 by French chemist M. Lebreton from the white inner layer of citrus peels (mesocarp, albedo).[2][3]
Hesperidin is believed to play a role in plant defense.
- ^ Dakshini KM (August 1991). "Hesperetin 7-rutinoside (hesperidin) and taxifolin 3-arabinoside as germination and growth inhibitors in soils associated with the weed, Pluchea lanceolata (DC) C.B. Clarke (Asteraceae)". Journal of Chemical Ecology. 17 (8): 1585–1591. Bibcode:1991JCEco..17.1585I. doi:10.1007/BF00984690. PMID 24257882. S2CID 35483504.
- ^ Lebreton, M (1828). "Sur la matière cristalline des orangettes, et analyse de ces fruits non encore developpés, famille des Hesperidées". Journal de Pharmacie et de Sciences Accessories. 14: 377. Archived from the original on 2020-10-20. Retrieved 2016-10-30.
- ^ "Metabocard for Hesperidin (HMDB03265)". Human Metabolome Database, The Metabolomics Innovation Centre, Genome Canada. 11 February 2016. Archived from the original on 30 October 2016. Retrieved 30 October 2016.