| Heterophile antibody test | |
|---|---|
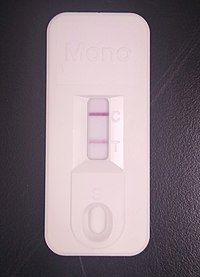 A commercial immunochromatographic test kit for the heterophile antibody test. Solid lines are visible at the "C" (control) and "T" (test) positions, indicating a positive result. | |
| Synonyms | Monospot test |
| Purpose | rapid test for infectious mononucleosis |
The mononuclear spot test or monospot test, a form of the heterophile antibody test,[1] is a rapid test for infectious mononucleosis due to Epstein–Barr virus (EBV). It is an improvement on the Paul–Bunnell test.[2] The test is specific for heterophile antibodies produced by the human immune system in response to EBV infection. Commercially available test kits are 70–92% sensitive and 96–100% specific, with a lower sensitivity in the first two weeks after clinical symptoms begin.[3][4]
The United States Center for Disease Control deems the monospot test not to be very useful.[5]
- ^ Basson V, Sharp AA (May 1969). "Monospot: a differential slide test for infectious mononucleosis". J. Clin. Pathol. 22 (3): 324–5. doi:10.1136/jcp.22.3.324. PMC 474075. PMID 5814738.
- ^ Seitanidis, B (1969). "A comparison of the Monospot with the Paul–Bunnell test in infectious mononucleosis and other diseases". J Clin Pathol. 22 (3): 321–3. doi:10.1136/jcp.22.3.321. PMC 474073. PMID 5814737.
- ^ Elgh, F; Linderholm, M (1996). "Evaluation of six commercially available kits using purified heterophile antigen for the rapid diagnosis of infectious mononucleosis compared with Epstein-Barr virus-specific serology". Clinical and Diagnostic Virology. 7 (1): 17–21. doi:10.1016/S0928-0197(96)00245-0. PMID 9077426.
- ^ Ebell, MH (1 October 2004). "Epstein–Barr virus infectious mononucleosis". American Family Physician. 70 (7): 1279–87. PMID 15508538.
- ^ "Epstein–Barr Virus and Infectious Mononucleosis Laboratory Testing". CDC. January 7, 2014. Retrieved 10 August 2016.