| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexamethylbenzene | |
| Other names
1,2,3,4,5,6-Hexamethylbenzene
Mellitene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.616 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H18 | |
| Molar mass | 162.276 g·mol−1 |
| Appearance | White crystalline powder |
| Density | 1.0630 g cm−3 |
| Melting point | 165.6 ± 0.7 °C |
| Boiling point | 265.2 °C (509.4 °F; 538.3 K) |
| insoluble | |
| Solubility | acetic acid, acetone, benzene, chloroform, diethyl ether, ethanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
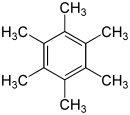
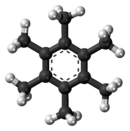
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat[1] and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.[2][3]
Hexamethylbenzene can be oxidised to mellitic acid,[4] which is found in nature as its aluminium salt in the rare mineral mellite.[5] Hexamethylbenzene can be used as a ligand in organometallic compounds.[6] An example from organoruthenium chemistry shows structural change in the ligand associated with changes in the oxidation state of the metal centre,[7][8] though the same change is not observed in the analogous organoiron system.[7]
In 2016 the crystal structure of the hexamethylbenzene dication C
6(CH
3)2+
6 was reported in Angewandte Chemie International Edition,[9] showing a pyramidal structure in which a single carbon atom has a bonding interaction with six other carbon atoms.[10][11] This structure was "unprecedented",[9] as the usual maximum valence of carbon is four, and it attracted attention from New Scientist,[10] Chemical & Engineering News,[11] and Science News.[12] The structure does not violate the octet rule since the carbon–carbon bonds formed are not two-electron bonds, and is pedagogically valuable for illustrating that a carbon atom "can [directly bond] with more than four atoms".[12] Steven Bachrach has demonstrated that the compound is hypercoordinated but not hypervalent, and also explained its aromaticity.[13] The idea of describing the chemical bonding in compounds and chemical species in this way through the lens of organometallic chemistry was proposed in 1975,[14] soon after the dication C
6(CH
3)2+
6 was first observed.[15][16][17]
- ^ Lonsdale, Kathleen (1929). "The Structure of the Benzene Ring in Hexamethylbenzene". Proc. R. Soc. A. 123 (792): 494–515. doi:10.1098/rspa.1929.0081.
- ^ Cite error: The named reference
Lydon1was invoked but never defined (see the help page). - ^ Cite error: The named reference
Lydon2was invoked but never defined (see the help page). - ^ Cite error: The named reference
HMBoxidationwas invoked but never defined (see the help page). - ^ Cite error: The named reference
mellitewas invoked but never defined (see the help page). - ^ Cite error: The named reference
AreneReviewwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Fewas invoked but never defined (see the help page). - ^ Cite error: The named reference
RuHMB2was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
PyramidalAngewChemwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
PyramidalNewSciwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
PyramidalC&ENewswas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
PyramidalSciNewswas invoked but never defined (see the help page). - ^ Cite error: The named reference
Bachrachwas invoked but never defined (see the help page). - ^ Cite error: The named reference
HMBviaOMetChemwas invoked but never defined (see the help page). - ^ Cite error: The named reference
HMBcatObs1was invoked but never defined (see the help page). - ^ Cite error: The named reference
HMBcatObs2was invoked but never defined (see the help page). - ^ Cite error: The named reference
HMBcatObs3was invoked but never defined (see the help page).