| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexamethylphosphoric triamide[3] | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| 1099903 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.595 |
| EC Number |
|
| 3259 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2810 3082 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H18N3OP | |
| Molar mass | 179.20 g/mol |
| Appearance | clear, colorless liquid[4] |
| Odor | aromatic, mild, amine-like[4] |
| Density | 1.03 g/cm3 |
| Melting point | 7.20 °C (44.96 °F; 280.35 K) |
| Boiling point | 232.5 °C (450.5 °F; 505.6 K) CRC[5] |
| miscible[4] | |
| Vapor pressure | 0.03 mmHg (4.0 Pa) at 20 °C[4] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Suspected Carcinogen[4] |
| GHS labelling: | |

| |
| Danger | |
| H340, H350 | |
| P201, P202, P281, P308+P313, P405, P501 | |
| Flash point | 104.4 °C (219.9 °F; 377.5 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[4] |
REL (Recommended)
|
Ca[4] |
IDLH (Immediate danger)
|
Ca [N.D.][4] |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
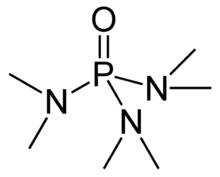
Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide (an amide of phosphoric acid) with the formula [(CH3)2N]3PO. This colorless liquid is a useful reagent in organic synthesis.
- ^ Not recommended: see Blue Book reference.
- ^ This name is also used to refer to tris(dimethylamino)phosphine
- ^ "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c d e f g h NIOSH Pocket Guide to Chemical Hazards. "#0321". National Institute for Occupational Safety and Health (NIOSH).
- ^ Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida, USA: CRC Press. p. 3-280. ISBN 978-1-43982077-3.