| |

| |
| Names | |
|---|---|
| IUPAC name
Tris[tetrammine-μ-dihydroxocobalt(III)]cobalt (III) ion
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Co4H42N12O18S3 | |
| Molar mass | 830.31 g·mol−1 |
| Sparingly soluble in water [1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
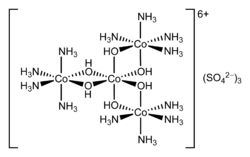
In chemistry, hexol is a cation with formula {[Co(NH3)4(OH)2]3Co}6+ — a coordination complex consisting of four cobalt cations in oxidation state +3, twelve ammonia molecules NH
3, and six hydroxy anions HO−
, with a net charge of +6. The hydroxy groups act as bridges between the central cobalt atom and the other three, which carry the ammonia ligands.
Salts of hexol, such as the sulfate {[Co(NH3)4(OH)2]3Co}(SO4)3(H2O)x, are of historical significance as the first synthetic non-carbon-containing chiral compounds.[2] [3]
- ^ Cite error: The named reference
InorgSynwas invoked but never defined (see the help page). - ^ Miessler, G. L. and Tarr, D. A. Inorganic Chemistry, 3rd ed., Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- ^ Ernst, Karl-Heinz; Berke, Heinz (2011). "Optical Activity and Alfred Werner's Coordination Chemistry". Chirality. 23 (3): 187–189. doi:10.1002/chir.20912. PMID 20928897.