| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Hydrogen azide
| |
| Other names
Hydrogen azide
Azoimide Azic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.059 |
| EC Number |
|
| 773 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HN3 | |
| Molar mass | 43.029 g·mol−1 |
| Appearance | colorless, highly volatile liquid |
| Density | 1.09 g/cm3 |
| Melting point | −80 °C (−112 °F; 193 K) |
| Boiling point | 37 °C (99 °F; 310 K) |
| highly soluble | |
| Solubility | soluble in alkali, alcohol, ether |
| Acidity (pKa) | 4.6 [1] |
| Conjugate base | Azide |
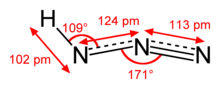
| Structure | |
| approximately linear | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Highly toxic, explosive, reactive |
| GHS labelling: | |
  
| |
| Danger | |
| H200, H319, H335, H370 | |
| P201, P202, P260, P261, P264, P270, P271, P280, P281, P304+P340, P305+P351+P338, P307+P311, P312, P321, P337+P313, P372, P373, P380, P401, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other cations
|
Sodium azide Lithium azide Potassium azide |
| Ammonia Hydrazine | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide,[2] is a compound with the chemical formula HN3.[3] It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3.[citation needed] It was first isolated in 1890 by Theodor Curtius.[4] The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
Hydrazoic acid, like its fellow mineral acids, is soluble in water. Undiluted hydrazoic acid is dangerously explosive[5] with a standard enthalpy of formation ΔfHo (l, 298K) = +264 kJ/mol.[6] When dilute, the gas and aqueous solutions (<10%) can be safely prepared but should be used immediately; because of its low boiling point, hydrazoic acid is enriched upon evaporation and condensation such that dilute solutions incapable of explosion can form droplets in the headspace of the container or reactor that are capable of explosion.[7][8]
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 3 (11th ed.). Cambridge University Press. pp. 82–83. This also contains a detailed description of the contemporaneous production process.
- ^ Dictionary of Inorganic and Organometallic Compounds. Chapman & Hall.
- ^ Curtius, Theodor (1890). "Ueber Stickstoffwasserstoffsäure (Azoimid) N3H" [On hydrazoic acid (azoimide) N3H]. Berichte der Deutschen Chemischen Gesellschaft. 23 (2): 3023–3033. doi:10.1002/cber.189002302232.
- ^ Furman, David; Dubnikova, Faina; van Duin, Adri C. T.; Zeiri, Yehuda; Kosloff, Ronnie (2016-03-10). "Reactive Force Field for Liquid Hydrazoic Acid with Applications to Detonation Chemistry". The Journal of Physical Chemistry C. 120 (9): 4744–4752. Bibcode:2016APS..MARH20013F. doi:10.1021/acs.jpcc.5b10812. ISSN 1932-7447. S2CID 102029987.
- ^ Cite error: The named reference
InorgChemwas invoked but never defined (see the help page). - ^ Gonzalez-Bobes, F. et al Org. Process Res. Dev. 2012, 16, 2051-2057.
- ^ Treitler, D. S. et al Org. Process Res. Dev. 2017, 21, 460-467.
