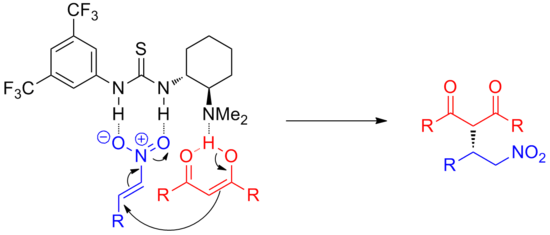
Hydrogen-bond catalysis is a type of organocatalysis that relies on use of hydrogen bonding interactions to accelerate and control organic reactions. In biological systems, hydrogen bonding plays a key role in many enzymatic reactions, both in orienting the substrate molecules and lowering barriers to reaction.[1] The field is relatively undeveloped compared to research in Lewis acid catalysis.[2]
Hydrogen-bond donors can catalyze reactions through a variety of mechanisms. Hydrogen bonding can stabilize anionic intermediates. They sequester anions, enabling the formation of reactive electrophilic cations. More acidic donors can act as general or specific acids, which activate electrophiles by protonation. A powerful approach is the simultaneous activation of both partners in a reaction, e.g. nucleophile and electrophile, termed "bifunctional catalysis". In all cases, the close association of the catalyst molecule to substrate also makes hydrogen-bond catalysis a powerful method of inducing enantioselectivity.
Hydrogen-bonding catalysts are often simple to make, relatively robust, and can be synthesized in high enantiomeric purity. New reactions catalyzed by hydrogen-bond donors are being discovered at an increasing pace, including asymmetric variants of common organic reactions, such as aldol additions, Diels-Alder cycloadditions and Mannich reactions.[3]
- ^ Jacobsen, E. N.; Knowles, R. R. (September 2010). "Attractive noncovalent interactions in asymmetric catalysis: Links between enzymes and small molecule catalysts" (PDF). Proc. Natl. Acad. Sci. 107 (48): 20678–20685. Bibcode:2010PNAS..10720678K. doi:10.1073/pnas.1006402107. PMC 2996434. PMID 20956302.
- ^ Jacobsen, E. N.; Taylor, M. S. (February 2006). "Asymmetric catalysis by chiral hydrogen-bond donors". Angew. Chem. Int. Ed. 45 (10): 1521–1539. doi:10.1002/anie.200503132. PMID 16491487.
- ^ Doyle, Abigail G.; Jacobsen, E. N. (December 2007). "Small-molecule H-bond donors in asymmetric catalysis". Chem. Rev. 107 (12): 5713–5743. doi:10.1021/cr068373r. PMID 18072808.