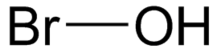
| |
 | |
| Names | |
|---|---|
| IUPAC name
Hypobromous acid
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.119.006 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HOBr | |
| Molar mass | 96.911 g·mol−1 |
| Density | 2.470 g/cm3 |
| Boiling point | 20–25 °C (68–77 °F; 293–298 K) |
| Acidity (pKa) | 8.65[1] |
| Conjugate base | Hypobromite |
| Related compounds | |
Other cations
|
Sodium hypobromite |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hypobromous acid is an inorganic compound with chemical formula of HOBr. It is a weak, unstable acid. It is mainly produced and handled in an aqueous solution. It is generated both biologically and commercially as a disinfectant. Salts of hypobromite are rarely isolated as solids.