| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphinic acid
| |||
| Other names
Hydroxy(oxo)-λ5-phosphane
Hydroxy-λ5-phosphanone | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.026.001 | ||
| KEGG | |||
PubChem CID
|
| ||
| UNII | |||
| UN number | UN 3264 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H3PO2 | |||
| Molar mass | 66.00 g/mol | ||
| Appearance | colorless, deliquescent crystals or oily liquid | ||
| Density | 1.493 g/cm3[2]
1.22 g/cm3 (50 wt% aq. solution) | ||
| Melting point | 26.5 °C (79.7 °F; 299.6 K) | ||
| Boiling point | 130 °C (266 °F; 403 K) decomposes | ||
| miscible | |||
| Solubility | very soluble in alcohol, ether | ||
| Acidity (pKa) | 1.2 | ||
| Conjugate base | Phosphinate | ||
| Structure | |||
| pseudo-tetrahedral | |||
| Hazards | |||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | JT Baker | ||
| Related compounds | |||
Related phosphorus oxoacids
|
Phosphorous acid Phosphoric acid | ||
Related compounds
|
Sodium hypophosphite Barium hypophosphite | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
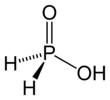
Hypophosphorous acid (HPA), or phosphinic acid, is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. It is a colorless low-melting compound, which is soluble in water, dioxane and alcohols. The formula for this acid is generally written H3PO2, but a more descriptive presentation is HOP(O)H2, which highlights its monoprotic character. Salts derived from this acid are called hypophosphites.[3]
HOP(O)H2 exists in equilibrium with the minor tautomer HP(OH)2. Sometimes the minor tautomer is called hypophosphorous acid and the major tautomer is called phosphinic acid.
- ^ Petrucci, Ralph H. (2007). General Chemistry (9th ed.). p. 946.
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.