| Icosahedrite | |
|---|---|
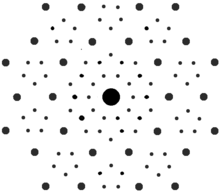 X-ray diffraction pattern of the natural Al63Cu24Fe13, icosahedrite. | |
| General | |
| Category | Native element mineral, alloy |
| Formula (repeating unit) | Al63Cu24Fe13 |
| IMA symbol | Ihd[1] |
| Crystal system | Quasicrystal |
| Space group | Icosahedral H-M symbol: 5 3m Space group: Fm 3 5[2] |
| Identification | |
| Color | Dark grey, black |
| Crystal habit | Subhedral to anhedral grains |
| Fracture | Irregular |
| Luster | Metallic |
| Streak | Grey |
| Diaphaneity | Opaque |
| Optical properties | Isotropic |
| References | [3] |
Icosahedrite is the first known naturally occurring quasicrystal phase. It has the composition Al63Cu24Fe13 and is a mineral approved by the International Mineralogical Association in 2010.[2][4] Its discovery followed a 10-year-long systematic search by an international team of scientists led by Luca Bindi and Paul J. Steinhardt to find the first natural quasicrystal.[5]
It occurs as tiny grains in a small sample labelled "khatyrkite" (catalog number 46407/G, housed in The Museum of Natural History, University of Florence, Italy), collected from an outcrop of weathered serpentinite in the Khatyrka ultramafic zone of the Koryak-Kamchatka area, Koryak Mountains, Russia. The rock sample also contains spinel, diopside, forsterite, nepheline, sodalite, corundum, stishovite, khatyrkite, cupalite and an unnamed AlCuFe alloy. Evidence shows that the sample is actually extraterrestrial in origin, delivered to the Earth by a CV3 carbonaceous chondrite asteroid that dates back 4.5 Gya.[6][5] A geological expedition has identified the exact place of the original discovery and found more specimens of the meteorite.[7][8] The same Al-Cu-Fe quasicrystal phase had previously been created in the laboratory by Japanese experimental metallurgists in the late 1980s.[9]
The concept of quasicrystals — along with the term — was first introduced in 1984 by Steinhardt and Dov Levine, both then at the University of Pennsylvania. The first synthetic quasicrystal, a combination of aluminium and manganese, was reported in 1984 by Israeli materials scientist Dan Shechtman and colleagues at the U.S. National Institute of Standards and Technology, a finding for which Shechtman won the 2011 Nobel Prize for Chemistry.[10][11]
- ^ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ a b Bindi, L.; Paul J. Steinhardt; Nan Yao; Peter J. Lu (2011). "Icosahedrite, Al63Cu24Fe13, the first natural quasicrystal" (PDF). American Mineralogist. 96 (5–6): 928–931. Bibcode:2011AmMin..96..928B. doi:10.2138/am.2011.3758. S2CID 101152220. Archived from the original (PDF) on 2012-04-04. Retrieved 2011-10-05.
- ^ Mindat.org
- ^ Commission on New Minerals and Mineral Names, Approved as new mineral Archived 2012-03-20 at the Wayback Machine
- ^ a b Bindi, Luca; John M. Eiler; Yunbin Guan; Lincoln S. Hollister; Glenn MacPherson; Paul J. Steinhardt; Nan Yao (2012-01-03). "Evidence for the extraterrestrial origin of a natural quasicrystal". Proceedings of the National Academy of Sciences. 109 (5): 1396–1401. Bibcode:2012PNAS..109.1396B. doi:10.1073/pnas.1111115109. PMC 3277151. PMID 22215583.
- ^ Paul J., Steinhardt (2012-09-15). "Quasicrystals: a brief history of the impossible, paper presented at the conference "The Centennial of X-Ray Diffraction (1912–2012)", held at Accademia Nazionale dei Lincei in Roma on May 8 and 9, 2012" (PDF). Rend. Fis. Acc. Lincei. 24: 85–91. doi:10.1007/s12210-012-0203-3. S2CID 6500074. Archived from the original (PDF) on 2013-10-14. Retrieved 2012-09-22.
- ^ Nadia Drake, Prospecting for Quasicrystals, Science News, Print edition: Nov. 3, 2012; Vol.182 #9 (p. 24)/ Web edition: October 19, 2012
- ^ A second natural quasicrystal with a different (decagonal) structure has been identified in the samples, Bindi, Luca (2015). "Natural quasicrystal with decagonal symmetry". Scientific Reports. 5: 9111. Bibcode:2015NatSR...5.9111B. doi:10.1038/srep09111. PMC 4357871. PMID 25765857.
- ^ Tsai, An-Pang; Akihisa Inoue; Tsuyoshi Masumoto (1987-09-20). "A Stable Quasicrystal in Al-Cu-Fe System". Japanese Journal of Applied Physics. 26 (Part 2, No. 9): L1505–L1507. Bibcode:1987JaJAP..26L1505T. doi:10.1143/JJAP.26.L1505. ISSN 0021-4922. S2CID 98442801.
- ^ Extra-terrestrial Origin
- ^ "Impossible" crystal discovery