 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Primaxin |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a686013 |
| License data | |
| Pregnancy category |
|
| Routes of administration | IM, IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 20% |
| Metabolism | Renal |
| Elimination half-life | 38 minutes (children), 60 minutes (adults) |
| Excretion | Urine (70%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.058.831 |
| Chemical and physical data | |
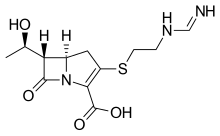
| Formula | C12H17N3O4S |
| Molar mass | 299.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Imipenem (trade name Primaxin among others) is a synthetic β-lactam antibiotic belonging to the carbapenems chemical class. developed by Merck scientists Burton Christensen, William Leanza, and Kenneth Wildonger in the mid-1970s.[1] Carbapenems are highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria,[2] thus playing a key role in the treatment of infections not readily treated with other antibiotics.[3] It is usually administered through intravenous injection.
Imipenem was patented in 1975 and approved for medical use in 1985.[4] It was developed via a lengthy trial-and-error search for a more stable version of the natural product thienamycin, which is produced by the bacterium Streptomyces cattleya. Thienamycin has antibacterial activity, but is unstable in aqueous solution, thus it is practically of no medicinal use.[5] Imipenem has a broad spectrum of activity against aerobic and anaerobic, Gram-positive and Gram-negative bacteria.[6] It is particularly important for its activity against Pseudomonas aeruginosa and Enterococcus species. However, it is not active against MRSA.
- ^ U.S. patent 4,194,047
- ^ Clissold SP, Todd PA, Campoli-Richards DM (March 1987). "Imipenem/cilastatin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy". Drugs. 33 (3): 183–241. doi:10.2165/00003495-198733030-00001. PMID 3552595. S2CID 209144637.
- ^ Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME (December 2012). "Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis". The Journal of Antimicrobial Chemotherapy. 67 (12): 2793–803. doi:10.1093/jac/dks301. PMID 22915465.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 497. ISBN 9783527607495.
- ^ Kahan FM, Kropp H, Sundelof JG, Birnbaum J (December 1983). "Thienamycin: development of imipenen-cilastatin". The Journal of Antimicrobial Chemotherapy. 12 Suppl D: 1–35. doi:10.1093/jac/12.suppl_d.1. PMID 6365872.
- ^ Kesado T, Hashizume T, Asahi Y (June 1980). "Antibacterial activities of a new stabilized thienamycin, N-formimidoyl thienamycin, in comparison with other antibiotics". Antimicrobial Agents and Chemotherapy. 17 (6): 912–7. doi:10.1128/aac.17.6.912. PMC 283902. PMID 6931548.