| Immunoglobulin domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
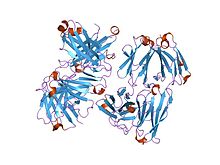 Structure of an antigen binding fragment of an antibody. | |||||||||
| Identifiers | |||||||||
| Symbol | ig | ||||||||
| Pfam | PF00047 | ||||||||
| Pfam clan | CL0159 | ||||||||
| InterPro | IPR013151 | ||||||||
| PROSITE | PDOC00262 | ||||||||
| SCOP2 | 8fab / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 193 | ||||||||
| OPM protein | 5f71 | ||||||||
| CDD | cd00096 | ||||||||
| Membranome | 2 | ||||||||
| |||||||||
The immunoglobulin domain, also known as the immunoglobulin fold, is a type of protein domain that consists of a 2-layer sandwich of 7-9 antiparallel β-strands arranged in two β-sheets with a Greek key topology,[1][2] consisting of about 125 amino acids.
The backbone switches repeatedly between the two β-sheets. Typically, the pattern is (N-terminal β-hairpin in sheet 1)-(β-hairpin in sheet 2)-(β-strand in sheet 1)-(C-terminal β-hairpin in sheet 2). The cross-overs between sheets form an "X", so that the N- and C-terminal hairpins are facing each other.
Members of the immunoglobulin superfamily are found in hundreds of proteins of different functions. Examples include antibodies, the giant muscle kinase titin, and receptor tyrosine kinases. Immunoglobulin-like domains may be involved in protein–protein and protein–ligand interactions.[3]
- ^ Bork P, Holm L, Sander C (September 1994). "The immunoglobulin fold. Structural classification, sequence patterns and common core". J. Mol. Biol. 242 (4): 309–20. doi:10.1006/jmbi.1994.1582. PMID 7932691.
- ^ Brümmendorf T, Rathjen FG (1995). "Cell adhesion molecules 1: immunoglobulin superfamily". Protein Profile. 2 (9): 963–1108. PMID 8574878.
- ^ Williams AF, Barclay AN (1988). "The immunoglobulin superfamily—domains for cell surface recognition". Annu. Rev. Immunol. 6: 381–405. doi:10.1146/annurev.iy.06.040188.002121. PMID 3289571.