| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1H-Indene[1] | |
| Other names
Benzocyclopentadiene
Indonaphthene Bicyclo[4.3.0]nona-1,3,5,7-tetraene | |
| Identifiers | |
3D model (JSmol)
|
|
| 635873 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.176 |
| EC Number |
|
| 27265 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H8 | |
| Molar mass | 116.16 |
| Appearance | Colorless liquid[2] |
| Density | 0.997 g/mL |
| Melting point | −1.8 °C (28.8 °F; 271.3 K) |
| Boiling point | 181.6 °C (358.9 °F; 454.8 K) |
| Insoluble | |
| Acidity (pKa) | 20.1 (in DMSO)[3] |
| −80.89×10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable |
| Flash point | 78.3 °C (172.9 °F; 351.4 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[2] |
REL (Recommended)
|
TWA 10 ppm (45 mg/m3)[2] |
IDLH (Immediate danger)
|
N.D.[2] |
| Related compounds | |
Related compounds
|
Benzofuran, Benzothiophene, Indole |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
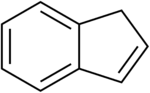
Indene is an aromatic, polycyclic hydrocarbon with chemical formula C9H8. It is composed of a benzene ring fused with a cyclopentene ring. This flammable liquid is colorless although samples often are pale yellow. The principal industrial use of indene is in the production of indene/coumarone thermoplastic resins. Substituted indenes and their closely related indane derivatives are important structural motifs found in many natural products and biologically active molecules, such as sulindac.[4]
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 207. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0340". National Institute for Occupational Safety and Health (NIOSH).
- ^ Bordwell FG (1988). "Equilibrium acidities in dimethyl sulfoxide solution". Accounts of Chemical Research. 21 (12): 456–463. doi:10.1021/ar00156a004. Bordwell pKa Table in DMSO Archived 2008-10-09 at the Wayback Machine
- ^ Wu, Jie; Qiu, Guanyinsheng (2014). "Generation of Indene Derivatives by Tandem Reactions". Synlett. 25 (19): 2703–2713. doi:10.1055/s-0034-1379318.