| |
| Names | |
|---|---|
| Other names
Indium(III) nitride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.042.831 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| InN | |
| Molar mass | 128.83 g/mol |
| Appearance | black powder |
| Density | 6.81 g/cm3 |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) |
| hydrolysis | |
| Band gap | 0.65 eV (300 K) |
| Electron mobility | 3200 cm2/(V.s) (300 K) |
| Thermal conductivity | 45 W/(m.K) (300 K) |
Refractive index (nD)
|
2.9 |
| Structure | |
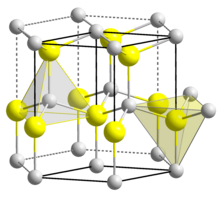
| Wurtzite (hexagonal) | |
| C46v-P63mc | |
a = 354.5 pm, c = 570.3 pm [1]
| |
| Tetrahedral | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant, hydrolysis to ammonia |
| Safety data sheet (SDS) | External SDS |
| Related compounds | |
Other anions
|
Indium phosphide Indium arsenide Indium antimonide |
Other cations
|
Boron nitride Aluminium nitride Gallium nitride |
Related compounds
|
Indium gallium nitride Indium gallium aluminium nitride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Indium nitride (InN) is a small bandgap semiconductor material which has potential application in solar cells[2] and high speed electronics.[3][4]
The bandgap of InN has now been established as ~0.7 eV depending on temperature[5] (the obsolete value is 1.97 eV). The effective electron mass has been recently determined by high magnetic field measurements,[6][7] m* =0.055 m0.
Alloyed with GaN, the ternary system InGaN has a direct bandgap span from the infrared (0.69 eV) to the ultraviolet (3.4 eV).
Currently there is research into developing solar cells using the nitride based semiconductors. Using one or more alloys of indium gallium nitride (InGaN), an optical match to the solar spectrum can be achieved.[citation needed] The bandgap of InN allows a wavelengths as long as 1900 nm to be utilized. However, there are many difficulties to be overcome if such solar cells are to become a commercial reality: p-type doping of InN and indium-rich InGaN is one of the biggest challenges. Heteroepitaxial growth of InN with other nitrides (GaN, AlN) has proved to be difficult.
Thin layers of InN can be grown using metalorganic chemical vapour deposition (MOCVD).[8]
- ^ Pichugin, I. G.; Tlachala, M. (1978). "Rentgenovsky analiz nitrida indiya" Рентгеновский анализ нитрида индия [X-ray analysis of indium nitride]. Izvestiya Akademii Nauk SSSR: Neorganicheskie Materialy Известия Академии наук СССР: Неорганические материалы (in Russian). 14 (1): 175–176.
- ^ Nanishi, Y.; Araki, T.; Yamaguchi, T. (2010). "Molecular-beam epitaxy of InN". In Veal, T. D.; McConville, C. F.; Schaff, W. J. (eds.). Indium Nitride and Related Alloys. CRC Press. p. 31. ISBN 978-1-138-11672-6.
- ^ Yim, J. W. L.; Wu, J. (2010). "Optical properties of InN and related alloys". In Veal, T. D.; McConville, C. F.; Schaff, W. J. (eds.). Indium Nitride and Related Alloys. CRC Press. p. 266. ISBN 978-1-138-11672-6.
- ^ Christen, Jürgen; Gil, Bernard (2014). "Group III nitrides". Physica Status Solidi C. 11 (2): 238. Bibcode:2014PSSCR..11..238C. doi:10.1002/pssc.201470041.
- ^ Monemar, B.; Paskov, P. P.; Kasic, A. (2005-07-01). "Optical properties of InN—the bandgap question". Superlattices and Microstructures. 38 (1): 38–56. Bibcode:2005SuMi...38...38M. doi:10.1016/j.spmi.2005.04.006. ISSN 0749-6036.
- ^ Goiran, Michel; Millot, Marius; Poumirol, Jean-Marie; Gherasoiu, Iulian; et al. (2010). "Electron cyclotron effective mass in indium nitride". Applied Physics Letters. 96 (5): 052117. Bibcode:2010ApPhL..96e2117G. doi:10.1063/1.3304169.
- ^ Millot, Marius; Ubrig, Nicolas; Poumirol, Jean-Marie; Gherasoiu, Iulian; et al. (2011). "Determination of effective mass in InN by high-field oscillatory magnetoabsorption spectroscopy". Physical Review B. 83 (12): 125204. Bibcode:2011PhRvB..83l5204M. doi:10.1103/PhysRevB.83.125204.
- ^ Cite error: The named reference
inushimawas invoked but never defined (see the help page).