 | |
| Clinical data | |
|---|---|
| Trade names | Picato |
| Other names | PEP005, ingenol-3-angelate |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613008 |
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | not absorbed when used topically |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.214.695 |
| Chemical and physical data | |
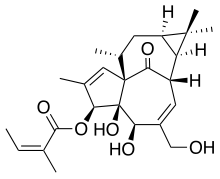
| Formula | C25H34O6 |
| Molar mass | 430.541 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ingenol mebutate, sold under the brand name Picato, is a substance that is found in the sap of the plant Euphorbia peplus,[1] commonly known as petty spurge, and is an inducer of apoptosis. This compound was isolated first from this plant in 2000.[2] A gel formulation of the drug has been approved by the U.S. Food and Drug Administration (FDA)[3] and by the European Medicines Agency (EMA)[4] for the topical treatment of actinic keratosis. Two different strengths of the gel have been approved for use on either the face and scalp (0.015%) or the trunk and extremities (0.05%), respectively.[5] In 2020 the drug was withdrawn from the market in the EU.[6]
- ^ Cite error: The named reference
Fallenwas invoked but never defined (see the help page). - ^ Hohmann J, Evanics F, Berta L, Bartók T (April 2000). "Diterpenoids from Euphorbia peplus". Planta Medica. 66 (3): 291–294. doi:10.1055/s-2000-8568. PMID 10821064. S2CID 260249228.
- ^ Cite error: The named reference
Drugs@FDAwas invoked but never defined (see the help page). - ^ Cite error: The named reference
EMAwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Approved labelwas invoked but never defined (see the help page). - ^ Cite error: The named reference
EMA-withdrawalwas invoked but never defined (see the help page).