
| |
| |
| |
| Names | |
|---|---|
| IUPAC name
myo-Inositol
| |
| Systematic IUPAC name
(1R,2S,3r,4R,5S,6s)-Cyclohexane-1,2,3,4,5,6-hexol | |
| Other names
cis-1,2,3,5-trans-4,6-Cyclohexanehexol
Cyclohexanehexol Mouse antialopecia factor Nucite Phaseomannite Phaseomannitol Rat antispectacled eye factor Scyllite (for the isomer scyllo-inositol) Vitamin B8 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.295 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g/mol |
| Density | 1.752 g/cm3 |
| Melting point | 225 to 227 °C (437 to 441 °F; 498 to 500 K) |
| Thermochemistry[2] | |
Std enthalpy of
formation (ΔfH⦵298) |
−1329.3 kJ/mol |
Std enthalpy of
combustion (ΔcH⦵298) |
−2747 kJ/mol |
| Pharmacology | |
| A11HA07 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 143 °C (289 °F; 416 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
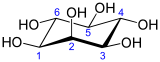
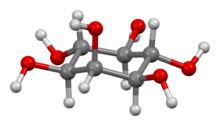
In biochemistry, medicine, and related sciences, inositol generally refers to myo-inositol (formerly meso-inositol), the most important stereoisomer of the chemical compound cyclohexane-1,2,3,4,5,6-hexol. Its formula is C6H12O6; the molecule has a ring of six carbon atoms, each with an hydrogen atom and a hydroxyl group (–OH). In myo-inositol, two of the hydroxyls, neither adjacent not opposite, lie above the respective hydrogens relative to the mean plane of the ring.
The compound is a carbohydrate, specifically a sugar alcohol (as distinct from aldoses like glucose) with half the sweetness of sucrose (table sugar). It is one of the most ancient components of living beings with multiple functions in eukaryotes, including structural lipids and secondary messengers.[3] A human kidney makes about two grams per day from glucose, but other tissues synthesize it too. The highest concentration is in the brain, where it plays an important role in making other neurotransmitters and some steroid hormones bind to their receptors.[4] In other tissues, it mediates cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and participates in osmoregulation.[5] In most mammalian cells the concentrations of myo-inositol are 5 to 500 times greater inside cells than outside them.[6]
A 2023 meta-analysis found that inositol is a safe and effective treatment in the management of polycystic ovary syndrome (PCOS).[7] However, there is only evidence of very low quality for its efficacy in increasing fertility for IVF in women with PCOS.[8]
The other naturally occurring stereoisomers of cyclohexane-1,2,3,4,5,6-hexol are scyllo-, muco-, D-chiro-, L-chiro-, and neo-inositol, although they occur in minimal quantities compared to myo-inositol. The other possible isomers are allo-, epi-, and cis-inositol.
- ^ Merck Index (11th ed.). p. 4883.
- ^ Knyazev, A.V.; Emel’yanenko, V.N.; Shipilova, A.S.; Zaitsau, D.H.; Lelet, M.I.; Knyazeva, S.S.; Gusarova, E.V.; Varfolomeev, M.A. (2018). "Thermodynamic properties of myo-inositol". The Journal of Chemical Thermodynamics. 116: 76–84. Bibcode:2018JChTh.116...76K. doi:10.1016/j.jct.2017.08.028. ISSN 0021-9614.
- ^ Cite error: The named reference
liyu2021was invoked but never defined (see the help page). - ^ Croze, M. L.; Soulage, C. O. (October 2013). "Potential role and therapeutic interests of myo-inositol in metabolic diseases". Biochimie. 95 (10): 1811–1827. doi:10.1016/j.biochi.2013.05.011. PMID 23764390.
- ^ Parthasarathy, L. K.; Seelan, R. S.; Tobias, C.; Casanova, M. F.; Parthasarathy, R. N. (2006). Mammalian inositol 3-phosphate synthase: its role in the biosynthesis of brain inositol and its clinical use as a psychoactive agent. Subcellular Biochemistry. Vol. 39. pp. 293–314. doi:10.1007/0-387-27600-9_12. ISBN 978-0-387-27599-4. PMID 17121280.
- ^ Cite error: The named reference
pmid33755975was invoked but never defined (see the help page). - ^ Cite error: The named reference
pcos2023was invoked but never defined (see the help page). - ^ Showell, M. G.; Mackenzie-Proctor, R.; Jordan, V.; Hodgson, R.; Farquhar, C. (2018). "Inositol for subfertile women with polycystic ovary syndrome". The Cochrane Database of Systematic Reviews. 2018 (12): CD012378. doi:10.1002/14651858.CD012378.pub2. PMC 6516980. PMID 30570133.
