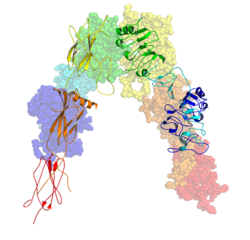
The insulin receptor (IR) is a transmembrane receptor that is activated by insulin, IGF-I, IGF-II and belongs to the large class of receptor tyrosine kinase.[5] Metabolically, the insulin receptor plays a key role in the regulation of glucose homeostasis; a functional process that under degenerate conditions may result in a range of clinical manifestations including diabetes and cancer.[6][7] Insulin signalling controls access to blood glucose in body cells. When insulin falls, especially in those with high insulin sensitivity, body cells begin only to have access to lipids that do not require transport across the membrane. So, in this way, insulin is the key regulator of fat metabolism as well. Biochemically, the insulin receptor is encoded by a single gene INSR, from which alternate splicing during transcription results in either IR-A or IR-B isoforms.[8] Downstream post-translational events of either isoform result in the formation of a proteolytically cleaved α and β subunit, which upon combination are ultimately capable of homo or hetero-dimerisation to produce the ≈320 kDa disulfide-linked transmembrane insulin receptor.[8]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000171105 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000005534 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Ward CW, Lawrence MC (April 2009). "Ligand-induced activation of the insulin receptor: a multi-step process involving structural changes in both the ligand and the receptor". BioEssays. 31 (4): 422–34. doi:10.1002/bies.200800210. PMID 19274663. S2CID 27645596.
- ^ Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauser E, Ou JH, Masiarz F, Kan YW, Goldfine ID (April 1985). "The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling". Cell. 40 (4): 747–58. doi:10.1016/0092-8674(85)90334-4. PMID 2859121. S2CID 23230348.
- ^ Malaguarnera R, Sacco A, Voci C, Pandini G, Vigneri R, Belfiore A (May 2012). "Proinsulin binds with high affinity the insulin receptor isoform A and predominantly activates the mitogenic pathway". Endocrinology. 153 (5): 2152–63. doi:10.1210/en.2011-1843. PMID 22355074.
- ^ a b Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R (October 2009). "Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease". Endocrine Reviews. 30 (6): 586–623. doi:10.1210/er.2008-0047. PMID 19752219.