 | |
| Clinical data | |
|---|---|
| Trade names | Omnipaque, Hexopaque, Oraltag, others |
| Other names | 5-[N-(2,3-Dihydroxypropyl)acetamido]-2,4,6-triiodo-N,N'-bis(2,3-dihydroxypropyl)isophthalamide |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Routes of administration | Intrathecal, intravascular, by mouth, intracavital, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Low |
| Metabolism | Nil |
| Elimination half-life | Variable |
| Excretion | Kidney, unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.060.130 |
| Chemical and physical data | |
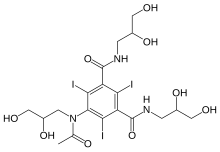
| Formula | C19H26I3N3O9 |
| Molar mass | 821.142 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 174 to 180 °C (345 to 356 °F) |
| |
| |
| | |
Iohexol, sold under the trade name Omnipaque among others, is a contrast agent used for X-ray imaging.[4] This includes when visualizing arteries, veins, ventricles of the brain, the urinary system, and joints, as well as during computed tomography (CT scan).[4] It is given by mouth, injection into a vein, or into a body cavity.[5]
Side effects include vomiting, skin flushing, headache, itchiness, kidney problems, and low blood pressure.[4] Less commonly allergic reactions or seizures may occur.[4] Allergies to povidone-iodine or shellfish do not affect the risk of side effects more than other allergies.[6] Use in the later part of pregnancy may cause hypothyroidism in the baby.[7] Iohexol is an iodinated non-ionic radiocontrast agent.[4] It is in the low osmolar family.[8]
Iohexol was approved for medical use in 1985.[9] It is on the World Health Organization's List of Essential Medicines.[10][5]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ "Regulatory Decision Summary for Omnipaque". Drug and Health Products Portal. 29 December 2023. Retrieved 2 April 2024.
- ^ a b c d e World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 317–8. hdl:10665/44053. ISBN 9789241547659.
- ^ a b Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 171. ISBN 9781284057560.
- ^ ACR Manual on Contrast Media v10.3. 2017 (PDF). American College of Radiology. 2017. p. 6. ISBN 9781559030120. Archived (PDF) from the original on 1 January 2018. Retrieved 1 January 2018.
- ^ Briggs GG, Freeman RK, Yaffe SJ (2011). Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. p. 761. ISBN 9781608317080. Archived from the original on 1 January 2017.
- ^ Sutton D, Young JW (2012). A Short Textbook of Clinical Imaging. Springer Science & Business Media. p. 235. ISBN 9781447117551. Archived from the original on 1 January 2017.
- ^ Broe ME, Porter GA, Bennett WM, Verpooten GA (2013). Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. Springer Science & Business Media. p. 325. ISBN 9789401590884. Archived from the original on 1 January 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.