 | |
| Clinical data | |
|---|---|
| Trade names | Marsilid, others |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 1 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.199 |
| Chemical and physical data | |
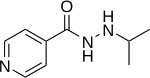
| Formula | C9H13N3O |
| Molar mass | 179.223 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.084 g/cm3 |
| Boiling point | 265.9 °C (510.6 °F) |
| |
| |
| (verify) | |
Iproniazid (Marsilid, Rivivol, Euphozid, Iprazid, Ipronid, Ipronin) is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class.[1][2] It is a xenobiotic that was originally designed to treat tuberculosis, but was later most prominently used as an antidepressant drug. However, it was withdrawn from the market because of its hepatotoxicity.[3][4] The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in France until its discontinuation in 2015.
- ^ Maxwell RA, Eckhardt SB (1990). "Iproniazid". Drug Discovery. Humana Press. pp. 143–154. ISBN 9780896031807.
- ^ Fagervall I, Ross SB (April 1986). "Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors". Biochemical Pharmacology. 35 (8): 1381–1387. doi:10.1016/0006-2952(86)90285-6. PMID 2870717.
- ^ Timbrell J (2008). Taylor & Francis Group. pp. 324–326. doi:10.3109/9781420007084. ISBN 978-0-8493-7302-2.
- ^ Henn F, Sartorius N, Helmchen H, Lauter H, eds. (2013-11-11). Contemporary Psychiatry. Springer Science & Business Media. p. 109. ISBN 9783642595196.