 α-IrCl3
| |
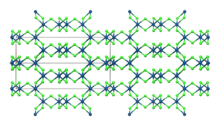 β-IrCl3
| |
 Iridium(III) chloride trihydrate
| |
| Names | |
|---|---|
| Other names
Iridium trichloride
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.028 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| IrCl3 | |
| Molar mass | 298.58 g/mol (anhydrous)
316.60 g/mol (hydrate) |
| Appearance | brown solid (α-anhydrous) red solid (β-anhydrous) dark green solid (trihydrate) |
| Density | 5.30 g/cm3, solid[1] |
| Melting point | 763 °C (1,405 °F; 1,036 K)[1][2] (decomposes) |
| insoluble (anhydrous IrCl3), soluble (hydrated derivative)[1] | |
| Solubility | Insoluble in HCl and alkanes[1] |
| −14.4·10−6 cm3/mol | |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-257 kJ/mol |
| Hazards | |
| GHS labelling:[3] | |
 
| |
| Warning | |
| H302, H411 | |
| Flash point | non-flammable |
| Related compounds | |
Other cations
|
Rhodium(III) chloride |
Related compounds
|
Platinum(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Iridium(III) chloride is the inorganic compound with the formula IrCl3. The anhydrous compound is relatively rare, but the related hydrate is much more commonly encountered. The anhydrous salt has two polymorphs, α and β, which are brown and red colored respectively. More commonly encountered is the hygroscopic dark green trihydrate IrCl3(H2O)3 which is a common starting point for iridium chemistry.[4]
- ^ a b c d Haynes, William, ed. (2014). CRC Handbook of Chemistry and Physics. CRC Press. p. 4-68. ISBN 9781482208689.
- ^ Cite error: The named reference
thermwas invoked but never defined (see the help page). - ^ "C&L Inventory". echa.europa.eu. Retrieved 23 December 2021.
- ^ Cite error: The named reference
housecroftwas invoked but never defined (see the help page).