| General | |
|---|---|
| Symbol | 56Fe |
| Names | iron-56, 56Fe, Fe-56 |
| Protons (Z) | 26 |
| Neutrons (N) | 30 |
| Nuclide data | |
| Natural abundance | 91.754% |
| Isotope mass | 55.9349375(7) Da |
| Spin | 0+ |
| Excess energy | −60601.003±1.354 keV |
| Binding energy | 492253.892±1.356 keV |
| Isotopes of iron Complete table of nuclides | |
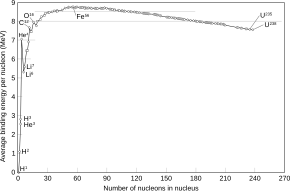
Iron-56 (56Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56.
Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei.[1]
The high nuclear binding energy for 56Fe represents the point where further nuclear reactions become energetically unfavorable. Because of this, it is among the heaviest elements formed in stellar nucleosynthesis reactions in massive stars. These reactions fuse lighter elements like magnesium, silicon, and sulfur to form heavier elements. Among the heavier elements formed is 56Ni, which subsequently decays to 56Co and then 56Fe.