| |
| |
| Names | |
|---|---|
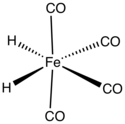
| Preferred IUPAC name
Tetracarbonyldihydridoiron(II)[citation needed] | |
| Other names
Iron tetracarbonyl dihydride, tetracarbonyldihydroiron
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| FeC 4H 2O 4 | |
| Molar mass | 169.901 g mol−1 |
| Appearance | Liquid (at -20 °C) |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | −20 °C (−4 °F; 253 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Iron tetracarbonyl dihydride is the organometallic compound with the formula H2Fe(CO)4. This compound was the first transition metal hydride discovered. The complex is stable at low temperatures but decomposes rapidly at temperatures above –20 °C.[1]