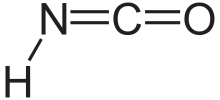
| |

| |
| Names | |
|---|---|
| IUPAC name
Isocyanic acid
| |
| Other names
Carbimide[1]
Carbonic imide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.109.068 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HNCO | |
| Molar mass | 43.025 g·mol−1 |
| Appearance | Colorless liquid or gas (boiling point near room temperature) |
| Density | 1.14 g/cm3 (20 °C) |
| Melting point | −86 °C (−123 °F; 187 K)[4] |
| Boiling point | 23.5 °C (74.3 °F; 296.6 K) |
| Dissolves | |
| Solubility | Soluble in benzene, toluene, diethyl ether |
| Acidity (pKa) | 3.7[2] |
| Conjugate acid | Oxomethaniminium[3] |
| Conjugate base | Cyanate |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Poisonous |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isocyanic acid is a chemical compound with the structural formula HNCO, which is often written as H−N=C=O. It is a colourless, volatile and poisonous substance, with a boiling point of 23.5 °C. It is the predominant tautomer and an isomer of cyanic acid (aka. cyanol) (H−O−C≡N).
The derived anion of isocyanic acid is the same as the derived anion of cyanic acid, and that anion is [N=C=O]−, which is called cyanate. The related functional group −N=C=O is isocyanate; it is distinct from cyanate (−O−C≡N), fulminate (−O−N+≡C−), and nitrile oxide (−C≡N+−O−).[5]
Isocyanic acid was discovered in 1830 by Justus von Liebig and Friedrich Wöhler.[6]
Isocyanic acid is the simplest stable chemical compound that contains carbon, hydrogen, nitrogen, and oxygen, the four most commonly found elements in organic chemistry and biology. It is the only fairly stable one of the four linear isomers with molecular formula HOCN that have been synthesized, the others being cyanic acid (cyanol, H−O−C≡N) and the elusive fulminic acid (H−C≡N+−O−)[7] and isofulminic acid H−O−N+≡C−.[5][8]
- ^ Cyanamide also has this name, and for which it is more systematically correct
- ^ Cite error: The named reference
scdbwas invoked but never defined (see the help page). - ^ "Oxomethaniminium | CH2NO | ChemSpider". www.chemspider.com. Retrieved 27 January 2019.
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ a b Martin, William R.; Ball, David W. (2019). "Small organic fulminates as high energy materials. Fulminates of acetylene, ethylene, and allene". Journal of Energetic Materials. 37 (1): 70–79. Bibcode:2019JEnM...37...70M. doi:10.1080/07370652.2018.1531089.
- ^ Liebig, J.; Wöhler, F. (1830). "Untersuchungen über die Cyansäuren". Ann. Phys. 20 (11): 394. Bibcode:1830AnP....96..369L. doi:10.1002/andp.18300961102.
- ^ Kurzer, Frederick (2000). "Fulminic Acid in the History of Organic Chemistry". Journal of Chemical Education. 77 (7): 851–857. Bibcode:2000JChEd..77..851K. doi:10.1021/ed077p851.
- ^ Quan, Donghui; Herbst, Eric; Osamura, Yoshihiro; Roueff, Evelyne (2010). "Gas-Grain Modeling of Isocyanic Acid (Hnco), Cyanic Acid (Hocn), Fulminic Acid (Hcno), and Isofulminic Acid (Honc) in Assorted Interstellar Environments". The Astrophysical Journal. 725 (2): 2101–2109. Bibcode:2010ApJ...725.2101Q. doi:10.1088/0004-637X/725/2/2101. hdl:1811/47796.