| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H7NO | |
| Molar mass | 73.095 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 59 °C (138 °F; 332 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
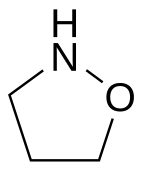
Isoxazolidine is the organic compound with the formula (CH2)3(NH)O. It is the parent of a family of compounds called Isoxazolidines, which are saturated C3NO heterocyclic rings where the nitrogen and oxygen occupy adjacent positions (1 and 2). They are the saturated analogues of Isoxazoles, and they are isomeric with oxazolidines, where the N and O are separated by one carbon.[1]
Isoxazolidines can be produced by the nitrone-olefin (3+2) cycloaddition reaction.
They represent precursors to 1,3-aminoalcohols.[2] The series Organic Syntheses provides detailed procedures that yield isoxazolidines, e.g., from styrene[3] and N-phenylmaleimide.[4] Some isoxazolidines are of medicinal interest.[5]
- ^ Cordero, Franca M.; Giomi, Donatella; Lascialfari, Luisa (2013). Five-Membered Ring Systems. Progress in Heterocyclic Chemistry. Vol. 25. pp. 291–317. doi:10.1016/B978-0-08-099406-2.00012-1. ISBN 9780080994062.
- ^ Frederickson, Martyn (1997). "Optically active isoxazolidines via asymmetric cycloaddition reactions of nitrones with alkenes: Applications in organic synthesis". Tetrahedron. 53 (2): 403–425. doi:10.1016/S0040-4020(96)01095-2.
- ^ Brüning, Ingrid; Grashey, Rudolf; Hauck, Hans; Huisgen, Rolf; Seidl, Helmut (1966). "2,3,5-Triphenylisoxazolidine". Organic Syntheses. 46: 127. doi:10.15227/orgsyn.046.0127.
- ^ Brüning, Ingrid; Grashey, Rudolf; Hauck, Hans; Huisgen, Rolf; Seidl, Helmut (1966). "2-phenyl-3-n-propylisoxazolidine-4,5-cis-dicarboxylic acid n-phenylimide". Organic Syntheses. 46: 96. doi:10.15227/orgsyn.046.0096.
- ^ Berthet, Mathéo; Cheviet, Thomas; Dujardin, Gilles; Parrot, Isabelle; Martinez, Jean (2016). "Isoxazolidine: A Privileged Scaffold for Organic and Medicinal Chemistry". Chemical Reviews. 116 (24): 15235–15283. doi:10.1021/acs.chemrev.6b00543. PMID 27981833.
