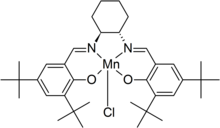
| |

| |
| Names | |
|---|---|
| IUPAC name
N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.108.565 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C36H52ClMnN2O2 | |
| Molar mass | 635.21 g·mol−1 |
| Appearance | brown solid |
| Melting point | 330 to 332 °C (626 to 630 °F; 603 to 605 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides.[1][2] Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation.[3] This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.
Enantiomerically pure epoxides are desirable as building blocks for complex molecules with specific chirality. Biologically active compounds can exhibit radically different activity based on differences in chirality and therefore the ability to obtain desired stereocenters in a molecule is of great importance to the pharmaceutical industry.[4] Jacobsen's catalyst and other asymmetric catalysts are particularly useful in this field; for example, Jacobsen's catalyst was used to synthesize phenylisoserine, a side chain to the famous anti-cancer drug Taxol, in a four-step synthesis as early as 1992.[5]
- ^ Zhang, W.; Loebach, J. L.; Wilson, S. R.; Jacobsen, E. N. (March 1990). "Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes". Journal of the American Chemical Society. 112 (7): 2801–2803. doi:10.1021/ja00163a052.
- ^ Jacobsen, Eric N.; Zhang, Wei; Muci, Alexander R.; Ecker, James R.; Deng, Li (1991). "Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane". Journal of the American Chemical Society. 113 (18): 7063. doi:10.1021/ja00018a068.
- ^ Robert H. Crabtree (2005). The Organometallic Chemistry of the Transition Metals. Wiley. pp. 405–408. ISBN 978-0-471-66256-3.
- ^ Caputo, CA; Jones, ND (2005). "Developments in Asymmetric Catalysis by Chiral Chelating Nitrogen-Donor Ligands". Dalton Transactions. 41 (41): 1563–1602. doi:10.1039/b709283k. PMID 17940641.
- ^ Deng, L; Jacobsen, EN (1992). "A Practical, Highly Enantioselective Synthesis of the Taxol Side Chain via Asymmetric Catalysis". J. Org. Chem. 57 (15): 4320–4323. doi:10.1021/jo00041a054.