| |
| |
| Names | |
|---|---|
| IUPAC name
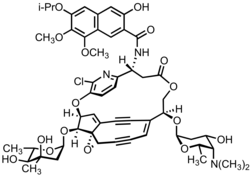
N-[(3S,9R,14S,15E,19S,21R,24R)-6-chloro-24-[(2S,4R,5S,6S)-4,5-dihydroxy-4,6-dimethyloxan-2-yl]oxy-14-[(2S,4S,5S,6S)-5-(dimethylamino)-4-hydroxy-6-methyloxan-2-yl]oxy-11-oxo-4,12,20-trioxa-7-azapentacyclo[13.6.2.25,8.13,21.019,21]hexacosa-1,5,7,15,25-pentaen-17,22-diyn-9-yl]-3-hydroxy-7,8-dimethoxy-6-propan-2-yloxynaphthalene-2-carboxamide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| KEGG |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C53H60ClN3O16 | |
| Molar mass | 1030.52 g·mol−1 |
| Appearance | Buff-colored amorphous solid |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Cytotoxic, mutagen |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Kedarcidin is a chromoprotein antitumor antibiotic first isolated from an Actinomycete in 1992, comprising an ansa-bridged enediyne chromophore (shown) as well as an apoprotein that serves to stabilize the toxin in the Actinomycete. Like other members of the enediyne class of drugs—so named for the nine-or-ten-membered core structure bearing an alkene directly attached to two alkynyl appendages—kedarcidin was likely evolved to kill bacteria that compete with the producing organism. Because it achieves this by causing DNA damage, however, kedarcidin is capable of harming tumor cells, as well. Kedarcidin is thus the subject of scientific research, both for its structural complexity as well as its anticancer properties.