 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Oruvail, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a686014 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99% |
| Elimination half-life | 2–2.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.676 |
| Chemical and physical data | |
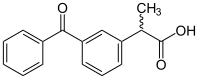
| Formula | C16H14O3 |
| Molar mass | 254.285 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Ketoprofen is one of the propionic acid class of nonsteroidal anti-inflammatory drugs (NSAID) with analgesic and antipyretic effects.[3] It acts by inhibiting the body's production of prostaglandin.
It was patented in 1967 and approved for medical use in 1980.[4]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ Kantor TG (1986). "Ketoprofen: a review of its pharmacologic and clinical properties". Pharmacotherapy. 6 (3): 93–103. doi:10.1002/j.1875-9114.1986.tb03459.x. PMID 3526298. S2CID 25309841.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 520. ISBN 9783527607495.