| |

| |
| Names | |
|---|---|
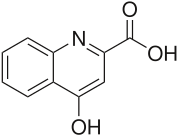
| Preferred IUPAC name
4-Hydroxyquinoline-2-carboxylic acid | |
| Other names
Kinurenic acid, kynuronic acid, quinurenic acid, transtorine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.047 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H7NO3 | |
| Molar mass | 189.168 g/mol |
| Melting point | 282.5 °C (540.5 °F; 555.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Kynurenic acid (KYNA or KYN) is a product of the normal metabolism of amino acid L-tryptophan. It has been shown that kynurenic acid possesses neuroactive activity. It acts as an antiexcitotoxic and anticonvulsant, most likely through acting as an antagonist at excitatory amino acid receptors. Because of this activity, it may influence important neurophysiological and neuropathological processes. As a result, kynurenic acid has been considered for use in therapy in certain neurobiological disorders. Conversely, increased levels of kynurenic acid have also been linked to certain pathological conditions.
Kynurenic acid was discovered in 1853 by the German chemist Justus von Liebig in dog urine, which it was apparently named after.[1]
It is formed from L-kynurenine in a reaction catalyzed by the enzyme kynurenine—oxoglutarate transaminase.[2]
- ^ Liebig, J., Uber Kynurensäure, Justus Liebigs Ann. Chem., 86: 125-126, 1853.
- ^ Han, Qian; Cai, Tao; Tagle, Danilo A.; Robinson, Howard; Li, Jianyong (August 2008). "Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II". Bioscience Reports. 28 (4): 205–215. doi:10.1042/BSR20080085. ISSN 0144-8463. PMC 2559858. PMID 18620547.