 | |
| Clinical data | |
|---|---|
| Trade names | Somatuline |
| Other names | Lanreotide acetate (JAN JP), Lanreotide acetate (USAN US) |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intramuscular, subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Approximately 80% |
| Protein binding | 78% |
| Metabolism | In GI tract |
| Elimination half-life | 2 hours (immediate release) 5 days (sustained release) |
| Excretion | Mostly bile duct |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.992 |
| Chemical and physical data | |
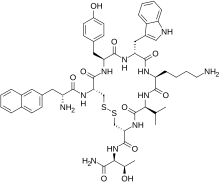
| Formula | C54H69N11O10S2 |
| Molar mass | 1096.33 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Lanreotide, sold under the brand name Somatuline among others, is a medication used in the management of acromegaly and symptoms caused by neuroendocrine tumors, most notably carcinoid syndrome. It is a long-acting analogue of somatostatin, like octreotide.
Lanreotide (as lanreotide acetate) is manufactured by Ipsen. It is available in several countries, including the United Kingdom, Australia and Canada, and was approved for sale in the United States by the Food and Drug Administration (FDA) on August 30, 2007.[2]
- ^ "Mytolac (Amdipharm Mercury Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 28 September 2022. Archived from the original on 13 November 2022. Retrieved 29 April 2023.
- ^ "FDA Approves New Drug to Treat Rare Disease, Acromegaly" (Press release). U.S. Food and Drug Administration. 30 August 2007. Archived from the original on 10 April 2021. Retrieved 6 September 2007.