| |
| Names | |
|---|---|
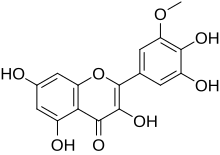
| IUPAC name
3,3′,4′,5,7-Pentahydroxy-5′-methoxyflavone
| |
| Systematic IUPAC name
2-(3,4-Dihydroxy-5-methoxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one | |
| Other names
3'-O-Methylmyricetin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O8 | |
| Molar mass | 332.264 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Laricitrin is an O-methylated flavonol, a type of flavonoid. It is found in red grape (absent in white grape)[1] and in Vaccinium uliginosum (bog bilberries).[2] It is one of the phenolic compounds present in wine.[3]
- ^ Mattivi, F; Guzzon, R; Vrhovsek, U; Stefanini, M; Velasco, R (October 2006). "Metabolite profiling of grape: Flavonols and anthocyanins". J. Agric. Food Chem. 54 (20): 7692–702. doi:10.1021/jf061538c. PMID 17002441.
- ^ Anja; Jaakola, Laura; Riihinen, Kaisu R.; Kainulainen, Pirjo S. (2010). "Anthocyanin and Flavonol Variation in Bog Bilberries (Vaccinium uliginosumL.) in Finland". Journal of Agricultural and Food Chemistry. 58 (1): 427–433. doi:10.1021/jf903033m. PMID 20000402.
- ^ Castillo-Munoz, Noelia; Gomez-Alonso, Sergio; Garcia-Romero, Esteban; Hermosin-Gutierrez, Isidro (2007). "Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines". Journal of Agricultural and Food Chemistry. 55 (3): 992–1002. doi:10.1021/jf062800k. PMID 17263504.