A lateral flow test (LFT),[1] is an assay also known as a lateral flow device (LFD), lateral flow immunochromatographic assay, or rapid test. It is a simple device intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment. LFTs are widely used in medical diagnostics in the home, at the point of care, and in the laboratory. For instance, the home pregnancy test is an LFT that detects a specific hormone. These tests are simple and economical and generally show results in around five to thirty minutes.[2] Many lab-based applications increase the sensitivity of simple LFTs by employing additional dedicated equipment.[3] Because the target substance is often a biological antigen, many lateral flow tests are rapid antigen tests (RAT or ART).
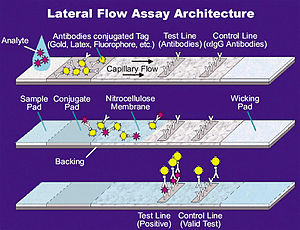
LFTs operate on the same principles of affinity chromatography as the enzyme-linked immunosorbent assays (ELISA). In essence, these tests run the liquid sample along the surface of a pad with reactive molecules that show a visual positive or negative result. The pads are based on a series of capillary beds, such as pieces of porous paper,[4] microstructured polymer,[5][6] or sintered polymer.[7] Each of these pads has the capacity to transport fluid (e.g., urine, blood, saliva) spontaneously.[8]
The sample pad acts as a sponge and holds an excess of sample fluid. Once soaked, the fluid flows to the second conjugate pad in which the manufacturer has stored freeze dried bio-active particles called conjugates (see below) in a salt–sugar matrix. The conjugate pad contains all the reagents required for an optimized chemical reaction between the target molecule (e.g., an antigen) and its chemical partner (e.g., antibody) that has been immobilized on the particle's surface. This marks target particles as they pass through the pad and continue across to the test and control lines. The test line shows a signal, often a color as in pregnancy tests. The control line contains affinity ligands which show whether the sample has flowed through and the bio-molecules in the conjugate pad are active. After passing these reaction zones, the fluid enters the final porous material, the wick, that simply acts as a waste container.
LFTs can operate as either competitive or sandwich assays.
- ^ Weiss, Alan (1 November 1999). "Concurrent engineering for lateral-flow diagnostics". IVD Technology. Archived from the original on 2014-04-15. Retrieved 1 October 2022.
- ^ Cite error: The named reference
Koczulawas invoked but never defined (see the help page). - ^ Yetisen AK, Akram MS, Lowe CR (June 2013). "Paper-based microfluidic point-of-care diagnostic devices". Lab on a Chip. 13 (12): 2210–51. doi:10.1039/C3LC50169H. PMID 23652632. S2CID 17745196.
- ^ "Lateral Flow Introduction". khufash.com. Archived from the original on 28 July 2012. Retrieved 27 July 2012.
- ^ Hansson J, Yasuga H, Haraldsson T, van der Wijngaart W (January 2016). "Synthetic microfluidic paper: high surface area and high porosity polymer micropillar arrays". Lab on a Chip. 16 (2): 298–304. doi:10.1039/C5LC01318F. PMID 26646057.
- ^ Weijin Guo; Jonas Hansson; Wouter van der Wijngaart (2016). "Viscosity Independent Paper Microfluidic Imbibition" (PDF). MicroTAS 2016, Dublin, Ireland.
- ^ Crozier, Alex; Rajan, Selina; Buchan, Iain; McKee, Martin (3 February 2021). "Put to the test: Use of rapid testing technologies for Covid-19". BMJ. 372: Appendix p. 6. doi:10.1136/bmj.n208. PMID 33536228. S2CID 231775752. Appendix Table 1, p. 6.
- ^ "Antibodies, Proteins, ELISA kits". Abbexa. Retrieved 12 January 2022.
Lateral Flow Assays, also known as Lateral Flow Immunochromatographic Assays [...] The technology is based on a series of capillary beds; such as pieces of porous paper, micro-structured polymer, or sintered polymer. Each of these elements has the capacity to transport fluid (e.g., urine), spontaneously.