| |
| Names | |
|---|---|
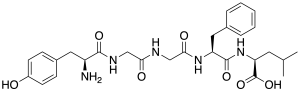
| IUPAC name
(2R)-2-[[(2R)-2-[[2-[[2-[[(2R)-2-amino-3-
| |
| Other names
[Leu]enkephalin; [Leu5]enkephalin;
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.055.852 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H37N5O7 | |
| Molar mass | 555.62 g/mol[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Leu-enkephalin is an endogenous opioid peptide neurotransmitter with the amino acid sequence Tyr-Gly-Gly-Phe-Leu that is found naturally in the brains of many animals, including humans.[2][3] It is one of the two forms of enkephalin; the other is met-enkephalin.[2] The tyrosine residue at position 1 is thought to be analogous to the 3-hydroxyl group on morphine.[4] Leu-enkephalin has agonistic actions at both the μ- and δ-opioid receptors, with significantly greater preference for the latter. It has little to no effect on the κ-opioid receptor.[5][6]
A nasal spray formulation of leu-enkephalin (developmental code names NES-100, NM-0127, NM-127, PES-200; proposed brand name Envelta) is under development by Virpax Pharmaceuticals for the treatment of pain and post-traumatic stress disorder (PTSD).[7] As of November 2023, it is up to the preclinical stage of development for these indications.[7]
- ^ Colaianni L, Kung SC, Taggart DK, Picca RA, Greaves J, Penner RM, Cioffi N (July 2014). "Reduction of spectral interferences using ultraclean gold nanowire arrays in the LDI-MS analysis of a model peptide". Analytical and Bioanalytical Chemistry. 406 (19): 4571–83. doi:10.1007/s00216-014-7876-7. PMID 24980599. S2CID 24046957.
- ^ a b Lazarus LH, Ling N, Guillemin R (June 1976). "beta-Lipotropin as a prohormone for the morphinomimetic peptides endorphins and enkephalins". Proceedings of the National Academy of Sciences of the United States of America. 73 (6): 2156–9. Bibcode:1976PNAS...73.2156L. doi:10.1073/pnas.73.6.2156. PMC 430469. PMID 1064883.
- ^ Hughes J, Kosterlitz HW, Smith TW (February 1997). "The distribution of methionine-enkephalin and leucine-enkephalin in the brain and peripheral tissues. 1977". British Journal of Pharmacology. 120 (4 Suppl): 428–36, discussion 426–7. doi:10.1111/j.1476-5381.1997.tb06829.x. PMC 3224324. PMID 9142421.
- ^ Schiller PW, Yam CF, Lis M (May 1977). "Evidence for topographical analogy between methionine-enkephalin and morphine derivatives". Biochemistry. 16 (9): 1831–8. doi:10.1021/bi00628a011. PMID 870028.
- ^ Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF (August 1984). "Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse". The Journal of Pharmacology and Experimental Therapeutics. 230 (2): 341–8. PMID 6086883.
- ^ Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T (February 1994). "Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors". Molecular Pharmacology. 45 (2): 330–4. PMID 8114680.
- ^ a b "Leucine enkephalin - Virpax Pharmaceuticals". AdisInsight. 20 November 2023. Retrieved 22 October 2024.