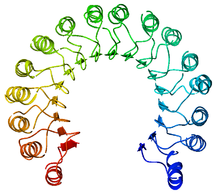
 An example of a leucine-rich repeat protein, a porcine ribonuclease inhibitor | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | LRR_1 | ||||||||
| Pfam | PF00560 | ||||||||
| Pfam clan | CL0022 | ||||||||
| ECOD | 207.1.1 | ||||||||
| InterPro | IPR001611 | ||||||||
| SCOP2 | 2bnh / SCOPe / SUPFAM | ||||||||
| Membranome | 605 | ||||||||
| |||||||||
| Leucine rich repeat variant | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 a leucine-rich repeat variant with a novel repetitive protein structural motif | |||||||||
| Identifiers | |||||||||
| Symbol | LRV | ||||||||
| Pfam | PF01816 | ||||||||
| Pfam clan | CL0020 | ||||||||
| InterPro | IPR004830 | ||||||||
| SCOP2 | 1lrv / SCOPe / SUPFAM | ||||||||
| Membranome | 737 | ||||||||
| |||||||||
| LRR adjacent | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 internalin h: crystal structure of fused n-terminal domains. | |||||||||
| Identifiers | |||||||||
| Symbol | LRR_adjacent | ||||||||
| Pfam | PF08191 | ||||||||
| InterPro | IPR012569 | ||||||||
| Membranome | 341 | ||||||||
| |||||||||
| Leucine rich repeat N-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 dimeric bovine tissue-extracted decorin, crystal form 2 | |||||||||
| Identifiers | |||||||||
| Symbol | LRRNT | ||||||||
| Pfam | PF01462 | ||||||||
| InterPro | IPR000372 | ||||||||
| SMART | LRRNT | ||||||||
| SCOP2 | 1m10 / SCOPe / SUPFAM | ||||||||
| Membranome | 127 | ||||||||
| |||||||||
| Leucine rich repeat N-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
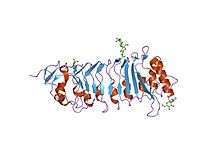 the crystal structure of pgip (polygalacturonase inhibiting protein), a leucine rich repeat protein involved in plant defense | |||||||||
| Identifiers | |||||||||
| Symbol | LRRNT_2 | ||||||||
| Pfam | PF08263 | ||||||||
| InterPro | IPR013210 | ||||||||
| SMART | LRRNT | ||||||||
| SCOP2 | 1m10 / SCOPe / SUPFAM | ||||||||
| |||||||||
| Leucine rich repeat C-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 third lrr domain of drosophila slit | |||||||||
| Identifiers | |||||||||
| Symbol | LRRCT | ||||||||
| Pfam | PF01463 | ||||||||
| InterPro | IPR000483 | ||||||||
| SMART | LRRCT | ||||||||
| SCOP2 | 1m10 / SCOPe / SUPFAM | ||||||||
| |||||||||
| LRV protein FeS4 cluster | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 a leucine-rich repeat variant with a novel repetitive protein structural motif | |||||||||
| Identifiers | |||||||||
| Symbol | LRV_FeS | ||||||||
| Pfam | PF05484 | ||||||||
| Pfam clan | CL0020 | ||||||||
| InterPro | IPR008665 | ||||||||
| SCOP2 | 1lrv / SCOPe / SUPFAM | ||||||||
| |||||||||
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold.[1][2] It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand-turn-alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues.
Leucine-rich repeats are frequently involved in the formation of protein–protein interactions.[3][4]
- ^ Kobe B, Deisenhofer J (October 1994). "The leucine-rich repeat: a versatile binding motif". Trends Biochem. Sci. 19 (10): 415–21. doi:10.1016/0968-0004(94)90090-6. PMID 7817399.
- ^ Enkhbayar P, Kamiya M, Osaki M, Matsumoto T, Matsushima N (February 2004). "Structural principles of leucine-rich repeat (LRR) proteins". Proteins. 54 (3): 394–403. doi:10.1002/prot.10605. PMID 14747988. S2CID 19951452.
- ^ Kobe B, Kajava AV (December 2001). "The leucine-rich repeat as a protein recognition motif". Curr. Opin. Struct. Biol. 11 (6): 725–32. doi:10.1016/S0959-440X(01)00266-4. PMID 11751054.
- ^ Gay NJ, Packman LC, Weldon MA, Barna JC (October 1991). "A leucine-rich repeat peptide derived from the Drosophila Toll receptor forms extended filaments with a beta-sheet structure". FEBS Lett. 291 (1): 87–91. doi:10.1016/0014-5793(91)81110-T. PMID 1657640. S2CID 84294221.