| |||
| Clinical data | |||
|---|---|---|---|
| Pronunciation | /lɛvɪtɪˈræsɪtæm/ | ||
| Trade names | Keppra, Elepsia, Spritam, others | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a699059 | ||
| License data | |||
| Pregnancy category |
| ||
| Routes of administration | By mouth, intravenous | ||
| Drug class | Racetam anticonvulsant | ||
| ATC code | |||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Bioavailability | ≈100% | ||
| Protein binding | <10% | ||
| Metabolism | Enzymatic hydrolysis of acetamide group | ||
| Elimination half-life | 6–8 hrs | ||
| Excretion | Kidney | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.121.571 | ||
| Chemical and physical data | |||
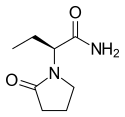
| Formula | C8H14N2O2 | ||
| Molar mass | 170.212 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Levetiracetam, sold under the brand name Keppra among others, is a medication used to treat epilepsy.[7] It is used for partial-onset, myoclonic, or tonic–clonic seizures and is taken either by mouth as an immediate or extended release formulation or by injection into a vein.[7]
Common side effects of levetiracetam include sleepiness, dizziness, feeling tired, and aggression.[7] Severe side effects may include psychosis, suicide, and allergic reactions such as Stevens–Johnson syndrome or anaphylaxis.[7] Levetiracetam is the S-enantiomer of etiracetam.[8] It acts as a synaptic vesicle glycoprotein 2A (SV2A) ligand.[9]
Levetiracetam was approved for medical use in the United States in 1999[7] and is available as a generic medication.[10] In 2022, it was the 123rd most commonly prescribed medication in the United States, with more than 5 million prescriptions.[11][12] It is on the World Health Organization's List of Essential Medicines.[13]
- ^ "Levetiracetam Use During Pregnancy". Drugs.com. Archived from the original on 6 March 2019. Retrieved 5 March 2019.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Keppra 100 mg/ml concentrate for solution for infusion - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 24 October 2021. Retrieved 9 September 2020.
- ^ Cite error: The named reference
Keppra tablet FDA labelwas invoked but never defined (see the help page). - ^ "Keppra XR- levetiracetam tablet, film coated, extended release". DailyMed. 4 November 2019. Archived from the original on 29 July 2021. Retrieved 9 September 2020.
- ^ "Keppra- levetiracetam injection, solution, concentrate". DailyMed. 4 November 2019. Archived from the original on 21 January 2016. Retrieved 9 September 2020.
- ^ a b c d e "Levetiracetam Monograph for Professionals". Drugs.com. AHFS. Archived from the original on 24 March 2019. Retrieved 14 January 2019.
- ^ Cavanna AE (2018). Behavioural Neurology of Anti-Epileptic Drugs: A Practical Guide. Oxford University Press. p. 17. ISBN 9780198791577.
- ^ Wu PP, Cao BR, Tian FY, Gao ZB (May 2024). "Development of SV2A Ligands for Epilepsy Treatment: A Review of Levetiracetam, Brivaracetam, and Padsevonil". Neurosci Bull. 40 (5): 594–608. doi:10.1007/s12264-023-01138-2. PMID 37897555.
- ^ British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 319. ISBN 9780857113382.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Levetiracetam Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.