 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Levo-Dromoran |
| Other names | Ro 1-5431[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682020 |
| Routes of administration | Oral, intravenous, subcutaneous, intramuscular |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70% (oral); 100% (IV) |
| Protein binding | 40% |
| Metabolism | Hepatic |
| Elimination half-life | 11–16 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.912 |
| Chemical and physical data | |
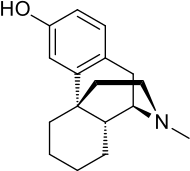
| Formula | C17H23NO |
| Molar mass | 257.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Levorphanol (brand name Levo-Dromoran) is an opioid medication used to treat moderate to severe pain.[1][3][4] It is the levorotatory enantiomer of the compound racemorphan. Its dextrorotatory counterpart is dextrorphan.
It was first described in Germany in 1946.[5] The drug has been in medical use in the United States since 1953.[6]
- ^ a b Elks J (November 14, 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 656–. ISBN 978-1-4757-2085-3.
- ^ Anvisa (March 31, 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published April 4, 2023). Archived from the original on August 3, 2023. Retrieved August 16, 2023.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 606–. ISBN 978-3-88763-075-1.
- ^ Morton IK, Hall JM (December 6, 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 165–. ISBN 978-94-011-4439-1.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 527. ISBN 978-3-527-60749-5.
- ^ Gudin J, Fudin J, Nalamachu S (January 2016). "Levorphanol Use: Past, Present and Future". Postgraduate Medicine. 128 (1): 46–53. doi:10.1080/00325481.2016.1128308. PMID 26635068. S2CID 3912175.