 | |
| Clinical data | |
|---|---|
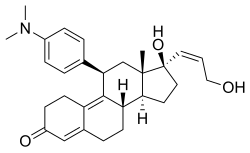
| Other names | ZK-98734; ZK-734; 11β-(4-(Dimethylamino)phenyl)-17β-hydroxy-17α-((Z)-3-hydroxypropenyl)estra-4,9-dien-3-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C29H37NO3 |
| Molar mass | 447.619 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lilopristone (INN) (developmental code names ZK-98734, ZK-734) is a synthetic, steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and was patented in 1985.[1][2][3][4] It is described as an abortifacient and endometrial contraceptive.[1][4][5] The drug differs from mifepristone only in the structure of its C17α side chain, and is said to have much reduced antiglucocorticoid activity in comparison.[6]
- ^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 733–. ISBN 978-1-4757-2085-3.
- ^ Rao KA (November 2009). Textbook of Gynaecology. Elsevier India. pp. 187–. ISBN 978-81-312-1526-5.
- ^ Baird DT, Schütz G, Krattenmacher R (9 March 2013). Organ-Selective Actions of Steroid Hormones. Springer Science & Business Media. pp. 108–. ISBN 978-3-662-09153-1.
- ^ a b Milne GW (8 May 2018). Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. pp. 23–. ISBN 978-1-351-78989-9.
- ^ Deshpande H (12 February 2016). Practical Management of Ovulation Induction. JP Medical Ltd. pp. 29–. ISBN 978-93-5250-028-4.
- ^ Van Look PF, Pérez-Palacios G, World Health Organization (1994). Contraceptive research and development, 1984 to 1994: the road from Mexico City to Cairo and beyond. Oxford University Press. p. 169. ISBN 978-0-19-563630-7.
[...] lilopristone, which differs from mifepristone only in the structure of the 17a side chain, is said to have a much reduced antiglucocorticoid activity (Neef et al., 1984).