 | |
| Clinical data | |
|---|---|
| Trade names | Linzess |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613007 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.243.239 |
| Chemical and physical data | |
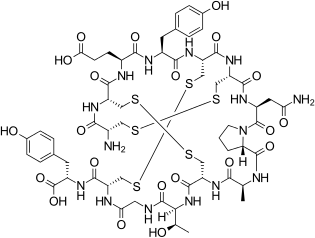
| Formula | C59H79N15O21S6 |
| Molar mass | 1526.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Linaclotide, (sold under the brand name Linzess in the US and Mexico, and as Constella elsewhere)[6] is a drug used to treat irritable bowel syndrome with constipation and chronic constipation with no known cause.[4][3] It has a black box warning about the risk of serious dehydration in children in the US; the most common adverse effects in others include diarrhea.[4]
It is an oligopeptide agonist of guanylate cyclase 2C and remains in the GI tract after it is taken by mouth. It was approved in the US and the European Union in 2012.[7]
It is marketed by Abbvie (formerly Allergan) in the United states and by Astellas in Asia;[citation needed] Ironwood Pharmaceuticals was the originator.[8][failed verification] In 2022, it was the 189th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[9][10]
- ^ Oh SA (17 August 2011). "Macrocycle Milestone for Ironwood Pharma". The Haystack. Archived from the original on 27 November 2018. Retrieved 11 February 2017 – via CENBlog.org.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b "UK label: Linaclotide Summary of Product Characteristics". Electronic Medicines Compendium. September 2017. Archived from the original on 15 April 2018. Retrieved 15 April 2018.
- ^ a b c "Linzess- linaclotide capsule, gelatin coated". DailyMed. 31 August 2021. Archived from the original on 29 March 2021. Retrieved 12 June 2023.
- ^ "Constella EPAR". European Medicines Agency. 24 May 2023. Archived from the original on 22 June 2021. Retrieved 12 June 2023.
- ^ "Linaclotide - Ironwood Pharmaceuticals". AdisInsight. Archived from the original on 7 October 2017. Retrieved 15 April 2018.
- ^ Yu SW, Rao SS (September 2014). "Advances in the management of constipation-predominant irritable bowel syndrome: the role of linaclotide". Therapeutic Advances in Gastroenterology. 7 (5): 193–205. doi:10.1177/1756283X14537882. PMC 4107700. PMID 25177366.
- ^ Nocera J (9 January 2018). "How Allergan Continues to Make Drug Prices Insane". Bloomberg News. Archived from the original on 15 April 2018. Retrieved 15 April 2018.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Linaclotide Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.