 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Vyvanse, Tyvense, Elvanse, others |
| Other names | L-Lysine-d-amphetamine; (2S)-2,6-Diamino-N-[(2S)-1-phenylpropan-2-yl]hexanamide N-[(2S)-1-Phenyl-2-propanyl]-L-lysinamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607047 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Moderate[1][2] |
| Addiction liability | Moderate[1][2] |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: 96.4%[9] |
| Protein binding | 20% (as dextroamphetamine)[10] |
| Metabolism | Hydrolysis by enzymes in red blood cells initially, subsequent metabolism follows |
| Metabolites | Dextroamphetamine (and its metabolites) and L-lysine |
| Onset of action | Oral: <2 hours[11][12] |
| Elimination half-life | Lisdexamfetamine: <1 hour[13] Dextroamphetamine: 10–12 h[13][7] |
| Duration of action | 10–14 hours[14][11][12] |
| Excretion | Kidney: ~2% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H25N3O |
| Molar mass | 263.385 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Dextrorotatory enantiomer |
| |
| |
| | |
Lisdexamfetamine, sold under the brand names Vyvanse and Elvanse among others, is a stimulant medication that is used to treat attention deficit hyperactivity disorder (ADHD) in children and adults and for moderate-to-severe binge eating disorder in adults.[15] Lisdexamfetamine is taken by mouth. Its effects generally begin within two hours and last for up to 14 hours.[15]
Common side effects of lisdexamfetamine include loss of appetite, anxiety, diarrhea, trouble sleeping, irritability, and nausea.[15] Rare but serious side effects include mania, sudden cardiac death in those with underlying heart problems, and psychosis.[15] It has a high potential for substance abuse.[7][15] Serotonin syndrome may occur if used with certain other medications.[15] Its use during pregnancy may result in harm to the baby and use during breastfeeding is not recommended by the manufacturer.[16][15][17]
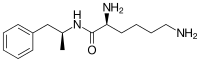
Lisdexamfetamine is an inactive prodrug that works after being converted by the body into dextroamphetamine, a central nervous system (CNS) stimulant.[15][18] Chemically, lisdexamfetamine is composed of the amino acid L-lysine, attached to dextroamphetamine.[19]
Lisdexamfetamine was approved for medical use in the United States in 2007, and in the European Union in 2012.[15][20] In 2022, it was the 69th most commonly prescribed medication in the United States, with more than 9 million prescriptions.[21][22] It is a Class B controlled substance in the United Kingdom, a Schedule 8 controlled drug in Australia, and a Schedule II controlled substance in the United States.[16][23]
- ^ a b "Adderall vs Vyvanse - What's the difference between them?". Drugs.com. Retrieved 12 March 2022.
- ^ a b Goodman DW (May 2010). "Lisdexamfetamine dimesylate (vyvanse), a prodrug stimulant for attention-deficit/hyperactivity disorder". P & T. 35 (5): 273–287. PMC 2873712. PMID 20514273.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Australian Product Information Vyanse® (Lisdexamfetamine dimesilate)" (PDF). Department of Health and Aged Care. Archived (PDF) from the original on 22 January 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 3 August 2023.
- ^ "Vyvanse Product information". Health Canada. 15 December 2021. Retrieved 18 March 2024.
- ^ a b c "Vyvanse- lisdexamfetamine dimesylate capsule; Vyvanse- lisdexamfetamine dimesylate tablet, chewable". DailyMed. 10 March 2022. Retrieved 19 December 2022.
- ^ "List of nationally authorised medicinal products : Active substance(s): lisdexamfetamine : Procedure No. PSUSA/00010289/202002" (PDF). Ema.europa.eu. Retrieved 12 March 2022.
- ^ "Public Assessment Report Decentralised Procedure" (PDF). MHRA. p. 14. Archived from the original (PDF) on 26 August 2014. Retrieved 23 August 2014.
- ^ Wishart DS, Djombou Feunang Y, Guo AC, Lo EJ, Marcu A, Grant JR, et al. "Amphetamine | DrugBank Online". DrugBank. 5.0.
- ^ a b Millichap JG (2010). "Chapter 9: Medications for ADHD". In Millichap JG (ed.). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. p. 112. ISBN 978-1-4419-1396-8.
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h] - ^ a b Brams M, Mao AR, Doyle RL (September 2008). "Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder". Postgraduate Medicine. 120 (3): 69–88. doi:10.3810/pgm.2008.09.1909. PMID 18824827. S2CID 31791162.
Onset of efficacy was earliest for d-MPH-ER at 0.5 hours, followed by d, l-MPH-LA at 1 to 2 hours, MCD at 1.5 hours, d, l-MPH-OR at 1 to 2 hours, MAS-XR at 1.5 to 2 hours, MTS at 2 hours, and LDX at approximately 2 hours. ... MAS-XR, and LDX have a long duration of action at 12 hours postdose
- ^ a b Cite error: The named reference
pmid27021968was invoked but never defined (see the help page). - ^ Stahl SM (March 2017). "Lisdexamfetamine". Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. pp. 379–384. ISBN 978-1-108-22874-9.
- ^ a b c d e f g h i "Lisdexamfetamine Dimesylate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 15 April 2019.
- ^ a b British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 348–349. ISBN 978-0-85711-338-2.
- ^ "Lisdexamfetamine (Vyvanse) Use During Pregnancy". Drugs.com. Retrieved 16 April 2019.
- ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ Blick SK, Keating GM (2007). "Lisdexamfetamine". Paediatric Drugs. 9 (2): 129–135, discussion 136–138. doi:10.2165/00148581-200709020-00007. PMID 17407369. S2CID 260863254.
- ^ "Shire's ADHD amphetamine wins British backing". Reuters. 12 December 2012. Retrieved 14 December 2023.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Lisdexamfetamine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Drugs of Abuse (PDF). Drug Enforcement Administration • U.S. Department of Justice. 2017. p. 22. Retrieved 16 April 2019.