| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Lithium acetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.105 |
| EC Number |
|
| KEGG | |
| MeSH | C488804 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H3LiO2 | |
| Molar mass | 65.98 g·mol−1 |
| Appearance | crystal |
| Density | 1.26 g/cm3 |
| Melting point | 286 °C (547 °F; 559 K) |
| 45.0 g/100 mL[1] | |
| −34.0·10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
500 mg/kg (oral, mouse) |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
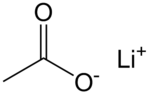
Lithium acetate (CH3COOLi) is a salt of lithium and acetic acid. It is often abbreviated as LiOAc.
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. p. 465. ISBN 0-8493-0594-2.
