
| |
 | |

| |
| Names | |
|---|---|
| IUPAC name
Lithium sulfate
| |
| Other names
Lithium sulphate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.734 |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| Li2SO4 | |
| Molar mass | 109.94 g/mol |
| Appearance | White crystalline solid, hygroscopic |
| Density | 2.221 g/cm3 (anhydrous) 2.06 g/cm3 (monohydrate) |
| Melting point | 859 °C (1,578 °F; 1,132 K) |
| Boiling point | 1,377 °C (2,511 °F; 1,650 K) |
| monohydrate: 34.9 g/100 mL (25 °C) 29.2 g/100 mL (100 °C) | |
| Solubility | insoluble in absolute ethanol, acetone and pyridine |
| −-40.0·10−6 cm3/mol | |
Refractive index (nD)
|
1.465 (β-form) |
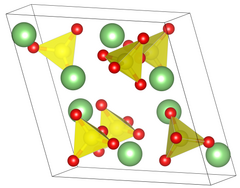
| Structure[2] | |
| Primitive monoclinic | |
| P 21/a, No. 14 | |
a = 8.239 Å, b = 4.954 Å, c = 8.474 Å α = 90°, β = 107.98°, γ = 90°[2]
| |
Lattice volume (V)
|
328.9 Å3 |
Formula units (Z)
|
4 |
| Tetrahedral at sulfur | |
| Thermochemistry | |
Heat capacity (C)
|
1.07 J/g K |
Std molar
entropy (S⦵298) |
113 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
−1436.37 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
-1324.7 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
613 mg/kg (rat, oral)[3] |
| Related compounds | |
Other anions
|
Lithium chloride |
Other cations
|
Sodium sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lithium sulfate is a white inorganic salt with the formula Li2SO4. It is the lithium salt of sulfuric acid.
- ^ Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8.
- ^ a b Nord, A. G. (1976). "Crystal structure of β-Li2SO4". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 32 (3): 982–983. doi:10.1107/S0567740876004433.
- ^ Chambers, Michael. "ChemIDplus - 10377-48-7 - INHCSSUBVCNVSK-UHFFFAOYSA-L - Lithium sulfate - Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.sis.nlm.nih.gov. Retrieved 12 October 2018.
