
In science, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond[1] and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom.
Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding pairs do not influence molecular geometry and are said to be stereochemically inactive. In molecular orbital theory (fully delocalized canonical orbitals or localized in some form), the concept of a lone pair is less distinct, as the correspondence between an orbital and components of a Lewis structure is often not straightforward. Nevertheless, occupied non-bonding orbitals (or orbitals of mostly nonbonding character) are frequently identified as lone pairs.
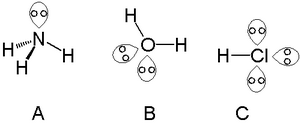
A single lone pair can be found with atoms in the nitrogen group, such as nitrogen in ammonia. Two lone pairs can be found with atoms in the chalcogen group, such as oxygen in water. The halogens can carry three lone pairs, such as in hydrogen chloride.
In VSEPR theory the electron pairs on the oxygen atom in water form the vertices of a tetrahedron with the lone pairs on two of the four vertices. The H–O–H bond angle is 104.5°, less than the 109° predicted for a tetrahedral angle, and this can be explained by a repulsive interaction between the lone pairs.[2][3][4]
Various computational criteria for the presence of lone pairs have been proposed. While electron density ρ(r) itself generally does not provide useful guidance in this regard, the Laplacian of the electron density is revealing, and one criterion for the location of the lone pair is where L(r) = –∇2ρ(r) is a local maximum. The minima of the electrostatic potential V(r) is another proposed criterion. Yet another considers the electron localization function (ELF).[5]
- ^ IUPAC Gold Book definition: lone (electron) pair
- ^ Fox, M.A.; Whitesell, J.K. (2004). Organic Chemistry. Jones and Bartlett Publishers. ISBN 978-0-7637-2197-8. Retrieved 5 May 2021.
- ^ McMurry, J. (2000). Organic Chemistry 5th Ed. Ceneage Learning India Pvt Limited. ISBN 978-81-315-0039-2. Retrieved 5 May 2021.
- ^ Lee, J.D. (1968). Concise Inorganic Chemistry. Student's paperback edition. Van Nostrand. Retrieved 5 May 2021.
- ^ Kumar, Anmol; Gadre, Shridhar R.; Mohan, Neetha; Suresh, Cherumuttathu H. (2014-01-06). "Lone Pairs: An Electrostatic Viewpoint". The Journal of Physical Chemistry A. 118 (2): 526–532. Bibcode:2014JPCA..118..526K. doi:10.1021/jp4117003. ISSN 1089-5639. PMID 24372481.