| |

| |
| Names | |
|---|---|
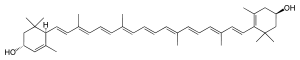
| IUPAC name
(3R,6R,3′R)-β,ε-Carotene-3,3′-diol
| |
| Systematic IUPAC name
(1R,4R)-4-{(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4R)-4-Hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl}-3,5,5-trimethylcyclohex-2-en-1-ol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.401 |
| E number | E161b (colours) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C40H56O2 | |
| Molar mass | 568.871 g/mol |
| Appearance | Red-orange crystalline solid |
| Melting point | 190 °C (374 °F; 463 K)[1] |
| Insoluble | |
| Solubility in fats | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lutein (/ˈljuːtiɪn, -tiːn/;[2] from Latin luteus meaning "yellow") is a xanthophyll and one of 600 known naturally occurring carotenoids. Lutein is synthesized only by plants, and like other xanthophylls is found in high quantities in green leafy vegetables such as spinach, kale and yellow carrots. In green plants, xanthophylls act to modulate light energy and serve as non-photochemical quenching agents to deal with triplet chlorophyll, an excited form of chlorophyll which is overproduced at very high light levels during photosynthesis. See xanthophyll cycle for this topic.
Animals obtain lutein by ingesting plants.[3] In the human retina, lutein is absorbed from blood specifically into the macula lutea,[4] although its precise role in the body is unknown.[3] Lutein is also found in egg yolks and animal fats.
Lutein is isomeric with zeaxanthin, differing only in the placement of one double bond. Lutein and zeaxanthin can be interconverted in the body through an intermediate called meso-zeaxanthin.[5] The principal natural stereoisomer of lutein is (3R,3′R,6′R)-beta,epsilon-carotene-3,3′-diol. Lutein is a lipophilic molecule and is generally insoluble in water. The presence of the long chromophore of conjugated double bonds (polyene chain) provides the distinctive light-absorbing properties. The polyene chain is susceptible to oxidative degradation by light or heat and is chemically unstable in acids.
Lutein is present in plants as fatty-acid esters, with one or two fatty acids bound to the two hydroxyl-groups. For this reason, saponification (de-esterification) of lutein esters to yield free lutein may yield lutein in any ratio from 1:1 to 1:2 molar ratio with the saponifying fatty acid.
- ^ MSDS at Carl Roth (Lutein Rotichrom, German).
- ^ "Lutein", Random House Webster's Unabridged Dictionary.
- ^ a b "Carotenoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis. July 2016. Retrieved 10 August 2017.
- ^ Bernstein, P. S.; Li, B; Vachali, P. P.; Gorusupudi, A; Shyam, R; Henriksen, B. S.; Nolan, J. M. (2015). "Lutein, Zeaxanthin, and meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-based Nutritional Interventions against Ocular Disease". Progress in Retinal and Eye Research. 50: 34–66. doi:10.1016/j.preteyeres.2015.10.003. PMC 4698241. PMID 26541886.
- ^ Krinksy, Norman; Landrum, John; Bone, Richard (2003). "Biological Mechanisms of the Protective Role of Lutein and Zeaxanthin in the Eye". Annual Review of Nutrition. 23 (1): 171–201. doi:10.1146/annurev.nutr.23.011702.073307. PMID 12626691.