 | |
| Clinical data | |
|---|---|
| Trade names | Lutathera |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Intravenous |
| Drug class | Radiolabeled somatostatin analog |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
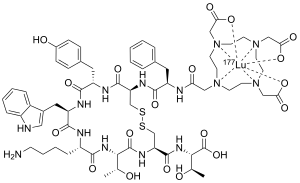
| Formula | C65H87LuN14O19S2 |
| Molar mass | 1607.58 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lutetium (177Lu) oxodotreotide (INN) or 177Lu dotatate, brand name Lutathera, is a chelated complex of a radioisotope of the element lutetium with dotatate, used in peptide receptor radionuclide therapy. Specifically, it is used in the treatment of cancers which express somatostatin receptors.[5] It is a radiolabeled somatostatin analog.[3][6][7]
Alternatives to 177Lu-dotatate include yttrium-90 dotatate or DOTATOC. The longer range of the beta particles emitted by 90Y, which deliver the therapeutic effect, may make it more suitable for large tumors with 177Lu reserved for smaller volumes[8][9]
The US Food and Drug Administration (FDA) considers 177Lu dotatate to be a first-in-class medication.[10]
- ^ "Summary Basis of Decision (SBD) for Lutathera". Health Canada. 23 October 2014. Archived from the original on 31 May 2022. Retrieved 29 May 2022.
- ^ "Lutathera 370 MBq/mL solution for infusion - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 9 July 2021. Retrieved 9 July 2021.
- ^ a b "Lutathera- lutetium lu 177 dotatate injection". DailyMed. 4 May 2020. Archived from the original on 16 November 2020. Retrieved 8 November 2020.
- ^ Cite error: The named reference
Lutathera EPARwas invoked but never defined (see the help page). - ^ Wang L, Tang K, Zhang Q, Li H, Wen Z, Zhang H, et al. (2013). "Somatostatin receptor-based molecular imaging and therapy for neuroendocrine tumors". BioMed Research International. 2013: 102819. doi:10.1155/2013/102819. PMC 3784148. PMID 24106690.
- ^ Cite error: The named reference
FDA PRwas invoked but never defined (see the help page). - ^ Cite error: The named reference
FDA PR 2was invoked but never defined (see the help page). - ^ Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. (January 2012). "Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs)". Gut. 61 (1): 6–32. doi:10.1136/gutjnl-2011-300831. PMC 3280861. PMID 22052063.
- ^ Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O'Dorisio MS, et al. (May 2013). "The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours". European Journal of Nuclear Medicine and Molecular Imaging. 40 (5): 800–16. doi:10.1007/s00259-012-2330-6. PMC 3622744. PMID 23389427.
- ^ New Drug Therapy Approvals 2018 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2019. Archived from the original on 17 September 2020. Retrieved 16 September 2020.