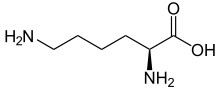
 Skeletal formula of L-lysine
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
L-lysine
D-lysine | |||
| Systematic IUPAC name
(2S)-2,6-Diaminohexanoic acid (L-lysine)
(2R)-2,6-Diaminohexanoic acid (D-lysine) | |||
| Other names
Lysine, D-lysine, L-lysine, LYS, h-Lys-OH
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.673 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H14N2O2 | |||
| Molar mass | 146.190 g·mol−1 | ||
| 1.5 kg/L | |||
| Pharmacology | |||
| B05XB03 (WHO) | |||
| Supplementary data page | |||
| Lysine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Lysine (symbol Lys or K)[2] is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated −NH+3 form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group (which is in the deprotonated −COO− form when the lysine is dissolved in water at physiological pH), and a side chain (CH2)4NH2 (which is partially protonated when the lysine is dissolved in water at physiological pH), and so it is classified as a basic, charged (in water at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.
The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct enzymes and substrates and are found in diverse organisms. Lysine catabolism occurs through one of several pathways, the most common of which is the saccharopine pathway.
Lysine plays several roles in humans, most importantly proteinogenesis, but also in the crosslinking of collagen polypeptides, uptake of essential mineral nutrients, and in the production of carnitine, which is key in fatty acid metabolism. Lysine is also often involved in histone modifications, and thus, impacts the epigenome. The ε-amino group often participates in hydrogen bonding and as a general base in catalysis. The ε-ammonium group (−NH+3) is attached to the fourth carbon from the α-carbon, which is attached to the carboxyl (−COOH) group.[3]
Due to its importance in several biological processes, a lack of lysine can lead to several disease states including defective connective tissues, impaired fatty acid metabolism, anaemia, and systemic protein-energy deficiency. In contrast, an overabundance of lysine, caused by ineffective catabolism, can cause severe neurological disorders.
Lysine was first isolated by the German biological chemist Ferdinand Heinrich Edmund Drechsel in 1889 from hydrolysis of the protein casein,[4] and thus named it Lysin, from Greek λύσις (lysis) 'loosening'.[5][6] In 1902, the German chemists Emil Fischer and Fritz Weigert determined lysine's chemical structure by synthesizing it.[7]
The one-letter symbol K was assigned to lysine for being alphabetically nearest, with L being assigned to the structurally simpler leucine, and M to methionine.[8]
- ^ a b Williams, P. A.; Hughes, C. E.; Harris, K. D. M (2015). "L-Lysine: Exploiting Powder X-ray Diffraction to Complete the Set of Crystal Structures of the 20 Directly Encoded Proteinogenic Amino Acids". Angew. Chem. Int. Ed. 54 (13): 3973–3977. doi:10.1002/anie.201411520. PMID 25651303.
- ^ "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature and symbolism for amino acids and peptides. Recommendations 1983". Biochemical Journal. 219 (2): 345–373. 15 April 1984. doi:10.1042/bj2190345. PMC 1153490. PMID 6743224.
- ^ Lysine. The Biology Project, Department of Biochemistry and Molecular Biophysics, University of Arizona.
- ^ Drechsel E (1889). "Zur Kenntniss der Spaltungsprodukte des Caseïns" [[Contribution] to [our] knowledge of the cleavage products of casein]. Journal für Praktische Chemie. 2nd series (in German). 39: 425–429. doi:10.1002/prac.18890390135. On p. 428, Drechsel presented an empirical formula for the chloroplatinate salt of lysine – C8H16N2O2Cl2·PtCl4 + H2O – but he later admitted that this formula was wrong because the salt's crystals contained ethanol instead of water. See: Drechsel E (1891). "Der Abbau der Eiweissstoffe" [The disassembly of proteins]. Archiv für Anatomie und Physiologie (in German): 248–278; Drechsel E (1877). "Zur Kenntniss der Spaltungsproducte des Caseïns" [Contribution] to [our] knowledge of the cleavage products of casein] (in German). pp. 254–260.
From p. 256:] " … die darin enthaltene Base hat die Formel C6H14N2O2. Der anfängliche Irrthum ist dadurch veranlasst worden, dass das Chloroplatinat nicht, wie angenommen ward, Krystallwasser, sondern Krystallalkohol enthält, … " ( … the base [that's] contained therein has the [empirical] formula C6H14N2O2. The initial error was caused by the chloroplatinate containing not water in the crystal (as was assumed), but ethanol … )
- ^ Vickery, Hubert Bradford.; Schmidt, Carl L. A. (1 October 1931). "The History of the Discovery of the Amino Acids". Chemical Reviews. 9 (2): 169–318. doi:10.1021/cr60033a001. ISSN 0009-2665.
- ^ Drechsel E (1891). "Der Abbau der Eiweissstoffe" [The disassembly of proteins]. Archiv für Anatomie und Physiologie (in German): 248–278.; Fischer E (1891). "Ueber neue Spaltungsproducte des Leimes" [On new cleavage products of gelatin]. Archiv für Anatomie und Physiologie (in German): 465–469.
From p. 469:] " … die Base C6H14N2O2, welche mit dem Namen Lysin bezeichnet werden mag, … " ( … the base C6H14N2O2, which may be designated with the name "lysine", … ) [Note: Ernst Fischer was a graduate student of Drechsel.]
- ^ Fischer E, Weigert F (1902). "Synthese der α,ε – Diaminocapronsäure (Inactives Lysin)" [Synthesis of α,ε-diaminohexanoic acid ([optically] inactive lysine)]. Berichte der Deutschen Chemischen Gesellschaft (in German). 35 (3): 3772–3778. doi:10.1002/cber.190203503211.
- ^ Saffran, M. (April 1998). "Amino acid names and parlor games: from trivial names to a one-letter code, amino acid names have strained students' memories. Is a more rational nomenclature possible?". Biochemical Education. 26 (2): 116–118. doi:10.1016/S0307-4412(97)00167-2.

