| |

| |
| Names | |
|---|---|
| IUPAC name
Magnesium acetate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.050 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Mg(CH3COO)2 | |
| Molar mass | 142.394 (anhydrous) 214.455 (tetrahydrate) |
| Appearance | White hygroscopic crystals |
| Density | 1.45 g/cm3 (tetrahydrate) |
| Melting point | 80 °C (176 °F; 353 K) (tetrahydrate) |
| Soluble | |
| −116.0·10−6 cm3/mol (+4 H2O | |
| Related compounds | |
Other cations
|
Calcium acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
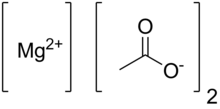
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2 and in its hydrated form, magnesium acetate tetrahydrate, it has the chemical formula Mg(CH3COO)2 • 4H2O. In this compound magnesium has an oxidation state of 2+. Magnesium acetate is the magnesium salt of acetic acid.[1] It is deliquescent and upon heating, it decomposes to form magnesium oxide.[2] Magnesium acetate is commonly used as a source of magnesium in biological reactions.[3]
- ^ Magnesium Acetate. Hazard.com. Retrieved on 2012-04-12.
- ^ Magnesium Acetate Supplier & Tech Info American Elements Retrieved on 2012-04-12.
- ^ "Sigma-Aldrich fact sheet on Magnesium acetate" (PDF). Retrieved 2012-04-26.