| |
| Names | |
|---|---|
| Other names
Sellaite
Irtran-1 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.086 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MgF2 | |
| Molar mass | 62.3018 g/mol |
| Appearance | Colorless to white tetragonal crystals |
| Density | 3.148 g/cm3 |
| Melting point | 1,263 °C (2,305 °F; 1,536 K) |
| Boiling point | 2,260 °C (4,100 °F; 2,530 K) |
| 0.013 g/(100 mL) | |
Solubility product (Ksp)
|
5.16⋅10−11 |
| Solubility |
|
| −22.7⋅10−6 cm3/mol | |
Refractive index (nD)
|
1.37397 |
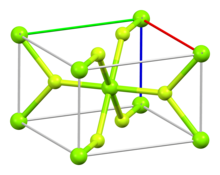
| Structure | |
| Rutile (tetragonal), tP6 | |
| P42/mnm, No. 136 | |
| Thermochemistry | |
Heat capacity (C)
|
61.6 J/(mol⋅K) |
Std molar
entropy (S⦵298) |
57.2 J/(mol⋅K) |
Std enthalpy of
formation (ΔfH⦵298) |
−1124.2 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
−1071 kJ/mol |
| Hazards[2][3] | |
| GHS labelling: | |

| |
| Warning | |
| H303, H315, H319, H335 | |
| P261, P304+P340, P305+P351+P338, P405 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2330[clarification needed] (rat, oral) |
| Safety data sheet (SDS) | ChemicalBook |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium fluoride is an ionically bonded inorganic compound with the formula MgF2. The compound is a colorless to white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs naturally as the rare mineral sellaite.
- ^ W.M. Haynes, ed. (2016), "Physical Constants of Inorganic Compounds", Handbook of Chemistry and Physics (97th ed.), CRC Press, pp. 4–71 (789), ISBN 978-1-4987-5429-3
- ^ "Magnesium Fluoride Material Safety Data Sheet". Science Labs. May 21, 2013. Retrieved October 13, 2017.
- ^ "Magnesium fluoride". CAS DataBase List. ChemicalBook. Retrieved October 13, 2017.
