
| |
| |
| Names | |
|---|---|
| IUPAC name
Magnesium hydroxide
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.013.792 |
| EC Number |
|
| E number | E528 (acidity regulators, ...) |
| 485572 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Mg(OH)2 | |
| Molar mass | 58.3197 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 2.3446 g/cm3 |
| Melting point | 350 °C (662 °F; 623 K) decomposes |
| |
Solubility product (Ksp)
|
5.61×10−12 |
| −22.1×10−6 cm3/mol | |
Refractive index (nD)
|
1.559[1] |
| Structure | |
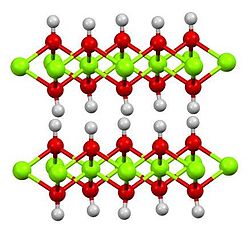
| Hexagonal, hP3[2] | |
| P3m1 No. 164 | |
a = 0.312 nm, c = 0.473 nm
| |
| Thermochemistry | |
Heat capacity (C)
|
77.03 J/mol·K |
Std molar
entropy (S⦵298) |
64 J·mol−1·K−1[3] |
Std enthalpy of
formation (ΔfH⦵298) |
−924.7 kJ·mol−1[3] |
Gibbs free energy (ΔfG⦵)
|
−833.7 kJ/mol |
| Pharmacology | |
| A02AA04 (WHO) G04BX01 (WHO) | |
| Hazards | |
| GHS labelling: | |
 [4] [4]
| |
| Warning[4] | |
| H315, H319, H335[4] | |
| P261, P280, P304+P340, P305+P351+P338, P405, P501[4] | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
8500 mg/kg (rat, oral) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
Magnesium oxide |
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (Ksp = 5.61×10−12).[5] Magnesium hydroxide is a common component of antacids, such as milk of magnesia.
- ^ Patnaik, Pradyot (2003). Handbook of inorganic chemicals. New York: McGraw-Hill. ISBN 0-07-049439-8. OCLC 50252041.
- ^ Toshiaki Enoki and Ikuji Tsujikawa (1975). "Magnetic Behaviours of a Random Magnet, NipMg(1−p)(OH)2". J. Phys. Soc. Jpn. 39 (2): 317–323. Bibcode:1975JPSJ...39..317E. doi:10.1143/JPSJ.39.317.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ a b c d "Magnesium Hydroxide". American Elements. Retrieved May 9, 2019.
- ^ Handbook of Chemistry and Physics (76th ed.). CRC Press. 12 March 1996. ISBN 0849305969.
