 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ludiomil, others |
| Other names | Maprotiline hydrochloride; Maprotiline methanesulfonate; Ba 34276[1][2][3] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682158 |
| Routes of administration | Oral, intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 66–70% |
| Protein binding | 88% |
| Metabolism | hepatic |
| Onset of action | 6 hours |
| Elimination half-life | 27–58 hours |
| Excretion | Urine (57%) and bile (30%) as glucuronides, 3–4% as unchanged drug |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.030.532 |
| Chemical and physical data | |
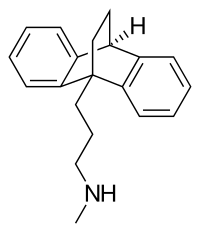
| Formula | C20H23N |
| Molar mass | 277.411 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Maprotiline, sold under the brand name Ludiomil among others, is a tetracyclic antidepressant (TeCA) that is used in the treatment of depression.[5] It may alternatively be classified as a tricyclic antidepressant (TCA), specifically a secondary amine.[5] In terms of its chemistry and pharmacology, maprotiline is closely related to such-other secondary-amine TCAs as nortriptyline and protriptyline and has similar effects to them,[6][5] albeit with more distinct anxiolytic effects.[7][8][9] Additionally, whereas protriptyline tends to be somewhat more stimulating and in any case is distinctly more-or-less non-sedating,[10] mild degrees of sedation may be experienced with maprotiline.[11]
- ^ Cite error: The named reference
Elks2014was invoked but never defined (see the help page). - ^ Cite error: The named reference
IndexNominum2000was invoked but never defined (see the help page). - ^ Cite error: The named reference
Drugs.comwas invoked but never defined (see the help page). - ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ a b c Cite error: The named reference
LemkeWilliams2012was invoked but never defined (see the help page). - ^ Cite error: The named reference
Zhou2013was invoked but never defined (see the help page). - ^ Guimarães FS, Zuardi AW, Graeff FG (January 1987). "Effect of chlorimipramine and maprotiline on experimental anxiety in humans". Journal of Psychopharmacology. 1 (3): 184–192. doi:10.1177/026988118700100305. PMID 22158980. S2CID 8444656.
- ^ Vinader-Caerols C, Martos AJ, Monleón S, Arenas MC, Parra A (2006). "Acute effects of maprotiline on learning, anxiety, activity and analgesia in male and female mice". Acta Neurobiologiae Experimentalis. 66 (1): 23–31. doi:10.55782/ane-2006-1584. PMID 16617674.
- ^ Pecknold JC, Familamiri P, McClure DJ, Elie R, Chang H (May 1985). "Trimipramine and maprotiline: antidepressant, anxiolytic, and cardiotoxic comparison". The Journal of Clinical Psychiatry. 46 (5): 166–171. PMID 2859273.
- ^ Brownell LG, Perez-Padilla R, West P, Kryger MH (1983). "The role of protriptyline in obstructive sleep apnea". Bulletin Européen de Physiopathologie Respiratoire. 19 (6): 621–4. PMID 6360257.
- ^ Holmberg G (May 1988). "Sedative effects of maprotiline and amitriptyline". Acta Psychiatrica Scandinavica. 77 (5): 584–6. doi:10.1111/j.1600-0447.1988.tb05171.x. PMID 3044007. S2CID 41977086.