 | |
| Clinical data | |
|---|---|
| Other names | Pyrilamine; N-[2-(dimethylamino)ethyl]-N-[(4-methoxyphenyl)methyl]pyridin-2-amine |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a606008 |
| Routes of administration | oral, topical, |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.912 |
| Chemical and physical data | |
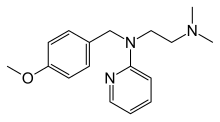
| Formula | C17H23N3O |
| Molar mass | 285.391 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Mepyramine, also known as pyrilamine, is a first generation antihistamine, targeting the H1 receptor as an inverse agonist.[1] Mepyramine rapidly permeates the brain, often causing drowsiness.[2] It is often sold as a maleate salt, pyrilamine maleate.
The medication has negligible anticholinergic activity, with 130,000-fold selectivity for the histamine H1 receptor over the muscarinic acetylcholine receptors (for comparison, diphenhydramine had 20-fold selectivity for the H1 receptor).[3]
It was patented in 1943 and came into medical use in 1949.[4] It was marketed under the names Histadyl, Histalon, Neo-Antergan, Neo-Pyramine, and Nisaval.[5] In the 1960s and 70s it was a very common component in over-the-counter sleep aids such as Alva-Tranquil, Dormin, Sedacaps, Sominex, Nytol, and many others.[5] The US FDA included it in the list of chemicals and compounds barred from use in over-the-counter nighttime sleep aid products in 1989.[6]
It is used in over-the-counter combination products to treat the common cold and menstrual symptoms such as Midol Complete.[7] It is also the active ingredient of the topical antihistamine creams Anthisan[8] and Neoantergan[1] sold for the treatment of insect bites, stings, and nettle rash.
- ^ a b Parsons ME, Ganellin CR (January 2006). "Histamine and its receptors". British Journal of Pharmacology. 147 (Suppl 1) (published 2 February 2009): S127–S135. doi:10.1038/sj.bjp.0706440. PMC 1760721. PMID 16402096.
- ^ "Mepyramine". drugbank.com. Retrieved 8 May 2021.
- ^ Kubo N, Shirakawa O, Kuno T, Tanaka C (March 1987). "Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay". Japanese Journal of Pharmacology. 43 (3): 277–282. doi:10.1254/jjp.43.277. PMID 2884340.
- ^ Fischer J, Gannelin CR, eds. (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 545. ISBN 9783527607495.
- ^ a b Thornton WE (September 1977). "Sleep aids and sedatives". Journal of the American College of Emergency Physicians (JACEP). 6 (9): 408–412. doi:10.1016/S0361-1124(77)80006-3. PMID 330911.
- ^ 54 FR 6826
- ^ "Active Ingredients for Midol Complete". Bayer HealthCare LLC. Archived from the original on 2 December 2009. Retrieved 8 December 2009.
- ^ "Anthisan Cream - Patient Information Leaflet (PIL)". Medicines.org.